Click here to download this post
The Brain-Gut Connection
Irritable and SIBO/Autonomic Dysfunction
Gastrointestinal (GI) symptoms and disorders are very common in the general population. The GI tract includes the Esophagus, Stomach, Small Intestine, and Large Intestine (including the Colon). Symptoms of the GI tract may include Gastro-Esophageal Reflux Disease (GERD), Gastroparesis, Crohn’s Disease, Irritable Bowel Syndrome (IBS), Constipation, or Diarrhea. Many patients who have various types of autonomic dysfunction also have significant GI complaints. Many patients with Hypermobility syndromes and Ehlers-Danlos Syndromes (EDS) likewise have accompanying GI disorders.
Two very common GI disorders, IBS and Small Intestinal Bacterial Overgrowth (SIBO), are underdiagnosed and are rather common and prevalent in the population. They are often associated with Autonomic Nervous System (ANS) disorders. The ANS includes the Parasympathetic and Sympathetic (P&S) Nervous Systems. Most consider GI disorders as accompanying comorbidities of ANS disorders. Some studies of patients with these disorders have shown abnormalities in both the peripheral ANS (outside the brain or spinal cord) and the Enteric-ANS (specifically the ANS in the gut, outside the spine). The Enteric Nervous System (ENS) is sometimes considered a part of the Parasympathetic Nervous System. It is also a major part of the “Gut Brain.” While the brain in your head has the largest collection of nerve cell bodies (the “decision-making” centers of nerves) in the human body, the collection of nerve cell bodies in the abdomen, or “gut,” is considerable; therefore, the title: “Gut Brain.” Recently there has been emphasis on a connection between IBS and SIBO. There has also been an association of GI permeability, also known as “the Leaky-Gut Syndrome,” and its association with IBS and SIBO, as well as with Celiac Disease.
IBS is very common and affects up to 20% of the population. Most patients can cope with this disorder without seeking medical care. However, about one fifth of the patients with IBS will have more significant symptoms that affect quality of life and ability to function. In addition, IBS is significantly more common among females than males. It may have a genetic and environmental component and a family history will often reveal other members of the family that have it.
IBS patients experience abdominal pain often times related to defecation and have altered bowel habits associated with change in the frequency or consistent of the stool. They should report pain at least one day a week in the last three months, while symptoms should exist for at least six months. There are specific criteria known as the Rome criteria[1] for diagnosis of IBS. There are forms of IBS that are predominantly associated with diarrhea, some with constipation and some with mixed. Bloating is often a common feature of IBS both with diarrhea and constipation types. Also, patients with IBS may have evidence of SIBO in which there is a 100,000-fold increase in bacteria growth over normal levels. That is 105 more colony-forming units per mL of gut aspirate.
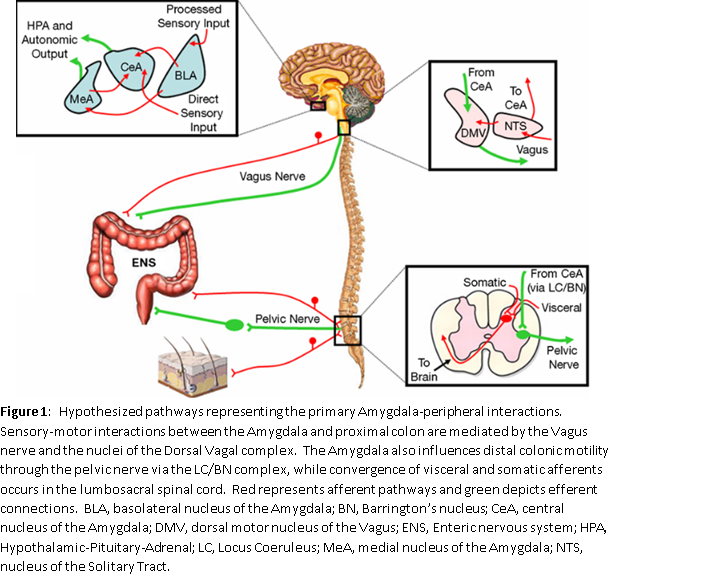
To better understand the association of autonomic dysfunction, IBS and SIBO we must first understand the brain-gut connection. This usually applies to dysfunction in the connection between the brain and the gut and is a major contributing factor to IBS. The ENS is the part of the ANS that is responsible for regulating the process of digestion. It manages motility, secretion of fluids and circulation to the GI tract. The ENS may operate at times independent of the peripheral ANS. There is a two-way communication with the CNS and the ENS (See Figure 1). By CNS, we are talking about the brain and the spine. Figure 1 represents part of the “brain-gut” connection. Abdominal pain, diarrhea, constipation, nausea, vomiting, flatulence, and abdominal distention are all symptoms of IBS. It is believed that there are nerves in the GI tract that experience hypersensitivity and could trigger changes in the brain that perceive this as discomfort. Many individuals who do not have these symptoms do not have this hypersensitivity of nerves in the GI tract which can trigger changes in the brain.
[1] At least three months, with onset at least six months previously, of recurrent abdominal pain or discomfort associated with two or more of the following: 1) Improvement with defecation, 2) Onset associated with a change in frequency of stool, or 3) Onset associated with a change in form (appearance) of stool. Discomfort means an uncomfortable sensation not described as pain.
Serotonin is a major neurotransmitter (a chemical that helps nerves communicate with each other and with the organs they control) in the gut as well as in the brain. In fact, it is believed that there is more Serotonin in the gut than in the brain. It appears that Serotonin does play a part in the Brain-Gut connection. Patients with diarrhea disorders have higher levels of serotonin in their blood following a meal, and those who have chronic constipation have lower levels of serotonin than normal in the blood after meals. This has led to the development of pharmacology which can affect various receptors in the GI tract which affects Serotonin. Note, since these receptors are more specific for the gut than anywhere else in the body, this Serotonin-based sub-system is what helps to differentiate the ENS from the Parasympathetic portion of the ANS.
Serotonin receptors 5-HT3 and 5-HT4 are targeted in treatment of IBS. For example, blockade of 5-HT3 can control diarrhea and stimulation of 5-HT4 can be used to treat chronic constipation. An example of a drug that is a 5-HT3 blocker is Lotronex, which is used for the treatment of diarrhea. 5-HT3 blockers are also useful in nausea. Zofran is an example of a 5-HT3 anti-nausea medication. Zelnorm is an example of a drug that is used to stimulate the 5-HT4 receptors and can increase motility in the GI tract and improve chronic constipation states.
Newer research now is looking at Serotonin Reuptake Transporters (SERTs) which are responsible for removing Serotonin when it is released. There are reflexes in the GI tract which can stimulate diarrhea, which can be trained through relaxation techniques to operate to the patients benefit. There is a Gastrocolic Reflex which causes the colon to contract after eating large meals or fatty foods. If a patient is constipated often, it is advantageous to eat a large fatty meal or a large meal. If the patient has significant diarrhea, it is often better to eat smaller meals at more frequent intervals to not over-activate the Gastrocolic Reflexes. The gut also has its own microbiome or bacterial inhabitance. These are known as gut microbes. These gut microbes can influence and perhaps communicate with the CNS. This is a type of symbiotic relationship between us and the bacteria in our environment. These bacteria communicate through several interacting channels involving nerves, hormones and immune signaling mechanisms. This helps maintain the Brain-Gut-microbiome access. This is a bidirectional interaction from the brain to the gut involving the microbiome. Alterations in this circuit can cause IBS and other functional GI disorders.
There is current evidence that the modulation of the CNS by the microbiome occurs primarily through neuroimmune and neuroendocrine mechanisms often involving the Vagus Nerve, which is the largest nerve in the body and is the majority of the Parasympathetic nervous system. There are molecules derived from the microbes which include short-chain fatty acids, secondary bile acids and other metabolites which participate in the communication and propagation of signals through various cells.
The Blood-Brain Barrier regulates molecule traffic between the circulatory system and cerebral spinal fluid. The Blood-Brain-Barrier separates the brain from the rest of the body to protect the electrical activities that must be kept very specific and very local to the individual cells of the brain. To this end, the Blood-Brain-Barrier has very tight junctions that prevent even the diffusion of water and salts into the brain. It has been discovered that gut microbiota can change the expression of tight junction proteins, causing the junctions to become even tighter and, thereby, decrease blood-brain barrier permeability. There is communication from the brain to the gut microbiota, and it is believed that social stressors can also reduce the relative proportions of bacteria in the GI tract. Both branches of the ANS regulate gut function including regional motilities (primarily a Parasympathetic function), secreting gastric acid by carbonate mucous and other Gastric secretions (like insulin, a Sympathetic function), antimicrobials, intestinal permeability and the immuno-response from the GI mucosa. Autonomic-induced changes in gut physiology affect the microbiota that inhabit the GI tract and affect its composition and their function. Intestinal transit time, overgrowth of bacteria and other factors can affect GI motility.
Stress can cause disruption of the epithelial barrier, the so called the “Leaky Gut,” and can cause the transportation of, or “leaking out” of, gut microbes or microbe-associated molecules into the blood stream. This can cause a proinflammatory state. Excess catecholamines can affect the mucous lining of the intestinal tract. This mucous lining is protective. Inflammation of the mucous lining can occur in stress and could change the microbiota composition.
Many functional intestinal disorders have reported significant microbial shifts in the GI tract. There may be various subgroups of patients with IBS who meet Rome criteria (see Footnote 1) based on different gut microbial populations. One can do analysis of fecal levels to assess the composition of the inhabitants of a microbial community. Stress alters the ANS modulation of the gut which then can affect the composition of the micro bacteria. Specifically, stress is mediated through the Sympathetic nervous system. As an example, Lactobacilli is a natural inhabitant of the human Gut that natural protects us against invasions of harmful bacteria. A reduction in Lactobacilli has been seen during stress. Stress causes increased autonomic-Sympathetic activation.
Therefore, one can see that communication between the brain and the gut involves the microbes that inhabit the gut and the composition of these microbes can change, and are affected by Sympathetic over-activation such as stress, high blood pressure, Anxiety, poor quality sleep, cardiovascular and respiratory diseases, lack of exercise, poor diet (lack of proper nutrients), etc.; and Parasympathetic over-activation such as depression, fatigue, and trauma (mental or physical); and many other factors that are altered by abnormal changes in the Parasympathetic and Sympathetic (P&S) nervous systems.
IBS is a disorder which has been very confusing, and there has been debate for years of whether it is organic (physiologic) or psychologic in origin. It is the most common GI disorder seen in the primary care physician’s office. There are three subtypes as mentioned, a diarrhea predominant, constipation predominant and a mixed diarrhea and constipation predominant. Increasing evidence is now giving support to the theory of dysregulation within the Brain-Gut axis, which we have just discussed. Also, by studying IBS we are learning more about the so-called “second brain” or “Gut-Brain” in the intestines, which is the Enteric autonomic nervous system. The altered bowel function pain, the abdominal pain, and the hypersensitivity that is seen in IBS results from disturbances in the interaction among the gut, the brain and the P&S nervous system.
Not enough has been studied or focused on the ENS. The ENS is an extensive network of neurons supported by enteric glia which are similar to Astrocytes in the brain. Glia are cells that wrap around the nerve cell projections called dendrites that connect the nerve cell bodies. Nerve cell bodies are the decision points like little micro-processors, connected to each other and to the organs by wires, the axons. These wires are wrapped in the glial cells, of which Astrocytes are one example, like the plastic coating on wires. They protect the wire inside and to help in conducting the signal down the wire. Again, like the brain, enteric glia may also enclose large bundles of enteric axons which are the portions of the neuron that transmit electric impulses. Interestingly, the ENS contains about 100,000, 000 nerve cells which approximately equals the number in the spinal cord. Therefore, this is a very complex neural organ.
The ENS comprises two large networks of autonomic nerves known as plexuses (see Figure 1). One plexus, the Myenteric Plexus, connects to and controls or coordinates the muscles of the intestine. It is responsible for motility and is the motor plexus conducting signals from the CNS to the ENS and within the ENS. There are muscles that wrap around the circumference of the intestines to (among other actions) churn the food in the small intestines and to squeeze out water in the large intestines. There are also muscles that run along the length of the intestines to move the contents from one end to the other like a conveyor belt. Among other things the signals from these longitudinal muscles (the “conveyor belt” muscles) help to empty the bowels as more food is digested, so wastes so not back up. This “conveyor belt” example demonstrates the interaction between the Myenteric Plexus and the second plexus referenced, the Submucosal Plexus. The combined action of the two sets of intestinal wall muscles is called “peristalsis.”
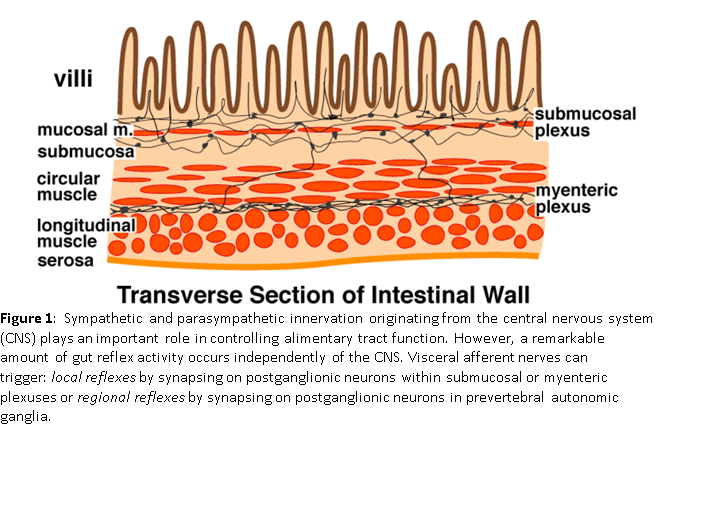
The Submucosal Plexus connects the ENS to the CNS as well as connects within the ENS. From the simple “conveyor belt” example demonstrates these connections. Within the ENS there are “reflexes” that work to move the contents of the intestines along in the proper direction. They are called reflexes because they are not initiated from within the brain. They function automatically. However, the CNS, including the brain, needs to “know” about this motion, if for nothing else to make sure you go to the bathroom in time. The Submucosal Plexus is the sensory plexus. It conducts signals from sensory cells just under the mucosa of the intestine to the rest of the ENS, including the Myenteric Plexus, and to the CNS. Among other functions, the signals from the Submucosal Plexus stimulate luminal secretions t just the right time and in just the right amount.
Signals from the brain to the gut are important for regulating digestive function and the various reflexes that occur in the GI tract and the effects of mood or psychological stress on the GI tract. The ENS is at times autonomous and can control peristalsis and motility in the GI tract and secretion within the GI tract lumen independent of the central nervous system, but we should not rule out the central nervous system can still modulate the ENS. For example, the “butterflies” in our stomach that we feel when nervous (like before a public speaking event or a musical or theatrical performance or before doing something very important to you) is a result of the effect that the CNS has on the ENS. This is a base reflex. The feeling in the stomach is designed to trigger an emptying of the bowels. This is to “lighten the load” in case you need to run away.
Again, the most important signaling molecule involved in the peristalsis reflex, or the muscle contraction motility of the GI tract is serotonin (5-HT). Almost all of the serotonin in the body is found in the GI tract. We have already discussed two serotonergic receptors that are targets of pharmacologic therapy, 5-HT3 and 5-HT4. The 5-HT3 receptors send signals indicating pain, nausea and other unpleasant sensations to the CNS. These 5-HT receptors are involved in GI, motor and secretory functions. The 5-HT4 receptors are help to promote peristalsis and chloride secretion and improve stool frequency and reduce bloating.
The agents that activate these selective 5-HT4 agonists (stimulants) have been beneficial in IBS with constipation in women. In patients with diarrhea, the 5-HT3 antagonists (blockers) are helpful in controlling diarrhea IBS patients with diarrhea. This is a complicated area but has led to development of pharmacology which can activate (stimulate) or block some of these Serotonin receptors and be beneficial in reducing symptoms of IBS of all three variants, including pain and bloating. In IBS with constipation patients, we favor starting with Psyllium and MiraLAX and adding, if need be, Lubiprostone or Linaclotide to better improve the motility, and at times, if needed, may even add Desipramine. Recently Motegrity (Prucalopride) was approved for IBS with constipation. It is a Serotonin agonist. In IBS with diarrhea patients, we may use low dose Loperamide or even Rifaximin, a non-absorbable antibiotic, and if need be add very low dose tricyclic antidepressants (TCAs) which can control the neural hypersensitivity of abdominal discomfort and diarrhea. TCAs are antidepressants at clinical doses (e.g., around 100 mg per day), but function as anticholinergics at low doses (of no more than 10 mg per day, and at the lower dose, the known side effects of antidepressants are present, including suicide risk). We also favor other antidepressants such as Selective Norepinephrine Reuptake Inhibitors (SNRIs) over Selective Serotonin Reuptake Inhibitors (SSRIs). For more advanced therapy, sometimes a drug called Alosetron is recommended. Alosetron works when a 5-HT4 mechanism is involved. Gastric bloating, including that associated with constipation, is difficult to treat in IBS. Low doses of high fermenting sugars and empiric Rifaximin have been advocated at times.
In summary, the pathophysiology of IBS is very complex. There is abundant data that supports visceral hypersensitivity alterations in the gut microbiome, intestinal permeability (“Leaky Gut Syndrome”), gut immune function and motility changes, brain-gut interactions, and psychosocial stress all may contribute to the development of IBS. Pain after eating meals reflects altered motility and may be an expression of a heightened Gastro-Colic Reflex. Increased permeability, which may be a mechanism in development of IBS will be discussed later under Leaky Bowel Syndrome where disrupted tight junctions between cells in the GI tract lead to increased permeability of toxins and bacterial products into the blood stream. This exposes the enteric nerves or nerves in the GI cells to stimuli which can cause the pain and discomfort in visceral hypersensitivity. After viral infections or gastroenteritis infections, IBS may actually emerge. Many feel it is the result of abnormalities that occur in the tight junctions between cells in the GI tract causing a “Leaky Gut.” Altered gut microbiota, as discussed earlier, may be associated with gut immune function and altered gut motility and can also lead to hypersensitivity in patients with IBS.
Again we should not discount psychosocial factors, as it has been shown that early life stress may cause development of exaggerated pain perception in patients with IBS, and we will often recommend stress relaxation techniques such as yoga and meditation or prayer to these patients.
Ultimately, IBS is a diagnosis of exclusion. While one has to fulfill the Rome criteria, or its definition, one really needs to exclude other more serious disorders and oftentimes a Gastroenterologist will exhaust many invasive and noninvasive tests in the process to exclude tumors, colitis, and infections. Note, the tests that take pictures of videos of the GI tract (Endoscopes, Colonoscopy, and the Smart Pill camera) assess the anatomy of the GI tract to look for blockages, etc. Motility disorders, such as IBS, also require physiologic tests to determine transit time and efficacy of peristalsis.
The first step in treating IBS in general, whether constipation or diarrhea predominates, is using a Low-FODMAP diet, which is a diet low in fermentable sugars (oligosaccharides and disaccharides). With this diet improvement in bloating, abdominal pain, and flatulence may improve, as well as the altered bowel movements. A dedicated diet needs to be adhered to in these cases. Gluten-free diets may also be effective, but there is debate over this issue. Soluble fiber seems to do a better job in the constipation-predominate IBS and antispasmodics, which include Dicyclomine and Hyoscyamine, and even Peppermint Oil, may be useful to relieve the abdominal pain and cramping. Antispasmodics, over some anticholinergics, may cause constipation and are preferably used in IBS with diarrhea. Antidepressants, especially tricyclics, may be useful in low dose. We already discussed IBS constipation specific medicines, Lubiprostone (Amitiza) and Linaclotide (Linzess). These are useful in constipation-predominant IBS. In diarrhea-predominant IBS, antidiarrheal agents may be used, bile acids such as Colestipol or Cholestyramine may be used, and even Welchol (which is used to lower cholesterol and also used to lower sugar in diabetes) Tricyclic antidepressants are more useful in diarrhea type of IBS including Amitriptyline, Nortriptyline, and Desipramine, although we will use Desipramine in constipation-predominant at times. Alosetron (Lotronex) blocks the extra serotonin 5-HT3 on the nerves in the GI tract and will slow the motility of the GI tract and improve diarrhea. It will also reduce pain and distention as well as flatus. However, side-effects such as Ischemic Colitis, perforation and death have been noted and therefore a GI specialist who is experienced in using it should prescribe the medication, in women specifically who have diarrhea predominant IBS.
Rifaximin (Xifaxan) is a nonabsorbable antibiotic, which has been studied in an IBS diarrhea-type in randomized trials and can be used in retreatment when flare-ups occur. However, it has side-effects of flatulence and abdominal pain and nausea, which may limit its use; also it is extremely expensive. One needs to seek more attention with the Gastroenterologist if they have uncontrolled IBS with constipation or diarrhea. Recently, an approved medication for diarrhea, Viberzi, is an antidiarrheal agent which operates through opiate receptors and may be useful.
Lastly, since there is a psychosocial component and a stress component which adversely affect the ANS and can aversely affect the microbiota in IBS patient, psychological interventions may be useful including cognitive behavior and relaxation techniques, such as yoga and meditation and prayer. Exercise and stress modulation are extremely important in treating patients. Acupuncture may also be helpful in some people, but there have been conflicting results. These activities help to normalize Parasympathetic activity which is a key factor.
There are two possible Parasympathetic effects: Parasympathetic Insufficiency and Parasympathetic Excess. Since the Parasympathetics, through the Vagus Nerve and the ENS, have the primary control of the GI tract, we should consider the interaction between the Parasympathetics and the GI tract for a moment. Parasympathetic Insufficiency indicates abnormally low Parasympathetic activity, which may cause abnormally slow GI motility, which may involve Gastroparesis and constipation. Parasympathetic Excess indicates abnormally high Parasympathetic activity, which may cause abnormally fast GI motility, which may involve diarrhea. Both may also cause Sympathetic Excess.
Consider a car, with brakes and an accelerator. The brakes are like the Parasympathetic nervous system and the accelerator is like the Sympathetic nervous system. In cases of Parasympathetic Insufficiency, motility is very low because there is no Parasympathetic activity to drive it, but with weak or no brakes, the accelerator (the Sympathetics) may be excessive in these cases as well, because there is no way to slow them. Therefore, Sympathetic symptoms including amplified pain, too much insulin, anxiety, and other stress symptoms predominate. With Parasympathetic Excess, it is like driving a car with a foot on the brakes. A foot on the brakes cases all accelerations to also be excessive just to get to normal speed; the engine is being over-revved to over-come the brakes. This is also Sympathetic Excess and may cause the same symptoms. The difference is whether there is motility or not. In cases of Parasympathetic Excess, the fact that at different times (changes in hormone levels, stress levels, emotional levels, exercise or diet, etc.) IBS may be associated with constipation or with diarrhea may be more understandable. When Parasympathetic Excess predominates, diarrhea is more likely. When the reactive Sympathetic Excess predominates, constipation is more likely. In both cases, getting the foot off the brakes helps to relieve the IBS and both diarrhea and constipation.
We should not discount the stress effect. During periods of increased Anxiety, hormones, such as Cortisol, Adrenalin and Serotonin are released by the brain, and this will raise the amount of Serotonin in your GI tract and cause stomach spasms to occur. If the spasms occur throughout your entire colon, an individual can get diarrhea because of a hypercontraction issue, but if the spasms are located to one area of the colon such as the sigmoid colon, one area which is extremely susceptible and which is almost considered an “Achilles heel,” of the GI tract, digestion may actually be curtailed and stopped and constipation may result. Therefore, a spasm which occurs with stress can be focal which can cause constipation or diffuse which can cause diarrhea and is usually mediated by high quantities of Serotonin in your gut after first being released from the brain.
Also, cortisol and adrenaline released from the brain can be adversely effective in times of stress. The stress, as mentioned, causes bacteria in the GI tract to become imbalanced. The term for this is Dysbiosis and may specifically contribute to IBS-related constipation. In more serious GI conditions, known as inflammatory bowel disease (IBD), stress can cause a flare up in these disorders. It is believed that chronic stress (a Sympathetic response) and depression (a Parasympathetic response), both occurring together as with Parasympathetic Excess, appear to increase inflammation (a Sympathetic Excess) which may set off the flares in IBD patients. Just as Anxiety (another Sympathetic Excess) may cause worsening of IBS or IBD flare-ups. Having these diseases and the comorbidities and symptoms associated with them may also cause Anxiety or more Anxiety, and this may cause a vicious cycle.
The cause of IBS for many years was thought to be largely psychogenic. However, now we know it is multifactorial. The identification of microbes which inhabit the GI tract and an imbalance which can occur with autonomic (Parasympathetic or Sympathetic) dysfunction and stress has been recognized to possibly cause gut Dysbiosis. Recently, a disorder known as Small Intestinal Bacterial Overgrowth has been noted to be associated with, or possibly cause, IBS symptoms. The fact that some probiotics and some absorbable and non-absorbable antibiotics may help improve symptoms in patients with IBS suggests that IBS may not originate in the brain but rather IBS may predominately originate in the GI tract (the ENS or the “Gut-Brain”) and supports a microbe altering basis for IBS.
SIBO is present when there is an increase in bacteria equal to, or greater than, 105 colony-forming units per mL in an upper gut aspirate test. These patients also experience abdominal pain, discomfort, bloating, flatulence, loose stools and may even have constipation, if one of the gasses secreted is methane and is in high quantities. It was once thought that SIBO only occurred in patients who have intestinal anatomical obstructions or malformations, but it is now realized that it may occur in the absence of anatomical factors predisposing to it. The gold standard for diagnosis is an aspirate from the small bowel and quantitating the amount of bacteria present. There are breath tests available; however, these can give false positives. These breath tests measure hydrogen, methane and hydrogen sulfide. They are simpler than doing a direct culture in the GI tract. However, as noted, there may be false-positives and the correlation with the gold standard stool cultures in the small bowel may not be precise. However, it is easy to follow patients with a breath test as these may be reproduced when therapy is given. For example, when an individual undergoes therapy for SIBO with antibiotics, one could measure a breath test and as it improves oftentimes the symptoms are improving and one knows that the SIBO is being treated appropriately. The two most commonly used substrates for testing for SIBO are Glucose and Lactulose. Lactulose, however, can cause diarrhea by itself and can hasten intestinal transit time; thereby, giving a false-positive breath test.
Testing for methane has become extremely important especially in identifying people who have chronic constipation disorders. The methane itself may slow intestinal transit and cause significant constipation. It is believed that microorganisms called Archaea, which are predominately found in the colon, may start populating the small intestine when there is significant methane gas present. The methane gas is produced by Archaea organisms. One can measure the bacteria Methanobrevibacter Smithii and correlate the levels of this bacteria with the degree of constipation individuals may have as this bacteria predominately secretes methane. The most common symptom with SIBO is bloating. More bacteria in the small bowel theoretically may cause a greater capacity for gas production and more abdominal distention and cramps. Flatulence and belching may become prominent.
There are many conditions associated with SIBO. These include motility disorders, such as gastroparesis, IBS, pseudo-obstruction, and constipation disorders known as colonic inertia, and mechanical abnormalities, such as adhesions and strictures, often seen with inflammatory bowel diseases, such as Crohn’s disease, bowel obstruction, polyps and tumors. Also Diabetes and Achlorhydria are sometimes associated with SIBO. Oftentimes, this is associated with Proton Pump Inhibitors (PPIs). There are immune mechanisms which may predispose to SIBO including deficiencies of IgA, collagen vascular diseases such as scleroderma and lupus, immunoglobulin abnormalities such as common variable immunodeficiency, HIV infections, chronic pancreatitis, acute pancreatitis episodes may also predispose to SIBO, as well as Cirrhosis. Ehlers-Danlos Syndrome has been known to predispose to SIBO. Medications such as opiates, anti-diarrheal agents, which slow GI transit, and acid-reducing agents specifically PPIs have been associated with SIBO. However, the most common disease associated with SIBO is IBS. This is often seen after an acute episode of Gastroenteritis where, especially females, can develop a new-onset of IBS and SIBO concurrently.
Besides IBS, conditions that have been associated with SIBO include Inflammatory Bowel Disease, Rosacea, Dyspepsia, Restless Leg Syndrome, Small Bowel Diverticula, Pancreatitis as noted, Hypothyroidism, Parkinson Disease, Diabetes, Coronary Artery Disease, abdominal surgery such as Hysterectomy, Cholecystectomy, Gastrectomy and Colectomy. SIBO is largely underdiagnosed. Risk factors for SIBO need to be sought for in taking a history of a patient with abdominal symptoms to potentially diagnosis more patients who have it. Again, low stomach acid, IBS symptoms, Celiac Disease, long-standing Crohn’s Disease, and other inflammatory bowel disease, prior bowel surgery, Diabetes Mellitus with type 1 and type 2, multiple courses of antibiotics, and organ dysfunction, such as Liver Cirrhosis, Chronic Pancreatitis or Renal Failure may predispose to SIBO along with abnormalities in the GI tract.
The goal of treating SIBO is symptom relief by eradicating any of the significant overgrowth of bacterial in trying to bring the GI flora back to a normal balance. Antibiotics are oftentimes the mainstay if diet is not effective. Sometimes the bacteria may be antibiotic resistant and there may be other underlying conditions, such as dysmotility or use of drugs such as PPIs that need to be sought after and corrected. One should treat predisposing conditions that cause SIBO. This is especially true if it was put in remission since it can recur. Promotility drugs and bowel preps with various types of laxatives to keep bowel motility functioning may be important in preventing the recurrence of SIBO. It is said that 44% of patients with SIBO may experience a relapse of symptoms within nine months of initial treatment. Avoiding medicines that delay gut transit time, improving glycemic control in diabetics, and correcting any anatomical abnormalities such as blind intestinal loops if they are present are the first line of treatment.
One should speak to their Gastroenterologist about using a prokinetic drug, even prophylactically so an occurrence does not recur. Many of these prokinetic drugs are the same ones that are used for gastroparesis or constipation and include Cisapride, Tegaserod, Erythromycin and the newly approved drug, Prucalopride. Some of these agents have risks and risk/benefit ratios have to be discussed with the physician. Some experts have even used low dose antibiotics rotating them cyclically every month and use two or three antibiotics that are known to be effective for these disorders. One should remember, however, that SIBO is not the explanation for all bloating abdominal pain or altered bowel habit. Whether positive for culture or negative for culture, it may be just small intestinal dysbiosis or an abnormal balance or other gut pathologies which may cause SIBO or be caused by SIBO.
Also, herbal microbials have been used as a good option to treat Colonic Dysbiosis and have fewer side-effects. The most successful antibiotic is the nonabsorbable antibiotic Rifaximin. Also, gut-directed stress management is important. Some patients have required a combination of Rifaximin and Neomycin, both nonabsorbable antibiotics, especially in constipation-predominant SIBO patients with methane-predominant bacterial overgrowth. Other antibiotics used have been Ciprofloxacin, Metronidazole, Amoxicillin-Clavulanic Acid and several others. Elemental diets have also been shown to be effective in 80% of patients with methane or hydrogen-predominant SIBO. These diets were originally developed for short bowel syndrome but now have extended use in patients with normal bowel structure who have SIBO. Interestingly, statins can also inhibit methane gas production directly and have been found to be useful in these patients.
Healthy exercise, proper diet and stress reduction are the most effective for long-term balance; both in the Gut Microbiome and in autonomic (P&S) balance – they go hand in hand. Furthermore, there is often no quick-fix. Both systems are like pendulums. They cannot be corrected with a sledge-hammer, it has to be gentle nudges over time. Quick fixes often push the balance too far in the opposite direction and lead to more or worsening symptoms.
Of course, with patients with SIBO, if there is an underlying disease it should be treated. For example, a flare-up of Crohn’s Disease or Celiac Disease should be treated directly along with SIBO. Diabetes should be treated, and avoidance of diabetic drugs known to slow the gut motility, such as Glucagon-like peptide 1-agonists should be avoided. We have found that patients with connective tissue disease and joint hypermobility syndromes (including EDS) benefit from pro-motility drugs. Therefore, treating SIBO and even patients with IBS who have not undergone testing for SIBO should concentrate on diets which can manipulate gut microbiota beneficially. Vegetarian diets rich in fiber lead to higher production of short chain fatty acids which inhibit potentially invasive bacteria like E. coli. Diets rich in complex carbohydrates favor a growth of less pathogenetic bacterium than diets rich in fat or protein.
The recent recognition that SIBO plays an important role in the pathogenesis of patients with IBS has led us away from a psychological etiology for the small bowel symptoms and disturbances. Many patients with concomitant autonomic dysfunction also have microbe abnormalities in the GI tract. SIBO is often associated with brain fogginess, and patients who receive different antibiotics report improvement of SIBO-related symptoms in over 70% of patients. This is further evidence of a physiologic and potential autonomic dysfunction. In many cases brain-fog is a function of poor brain perfusion associated with autonomic dysfunction. However, there is no agreement as to the frequency of SIBO among IBS patients. Some studies have shown as low as 4% and some as high as almost 80%. It is hoped that stool sampling with various immunological techniques may become more practical and sensitive and specific than small bowel culture or oral breath tests in the future. At the present time, Rifaximin is the best treatment for SIBO among patients with irritable bowel syndrome based on the totality of the data reviewed so far. Rifaximin should be prescribed by a Gastroenterologist and followed carefully as recurrences may need to be retreated.
One should not forget that there are other natural treatments besides diet such as probiotics, which include lactobacillus, Bifidobacterium, Saccharomyces, and other mixed compounds. In rare cases, SIBO may be precipitated by probiotics. However, as mentioned, this is extremely rare. Again, herbal supplements have also been proposed to be beneficial.
As mentioned, there is a brain-gut and microbiota miscommunication in patients with significant troubling GI symptoms. The peripheral ANS has been evaluated in many of these patients, although we now realize that the Enteric-ANS is also functioning independently especially with the transmitter Serotonin. If one looks at patients with IBS and constipation, one finds impaired Sympathetic activity and disturbed Parasympathetic function. It has been postulated that a central Sympathetic influence within the brain-gut axis is probably responsible for the myoelectrical activity disturbances in IBS patients [1]. These patients have increased Insulin, Norepinephrine and Epinephrine secretion; which are all results of Sympathetic activity.
IBS is associated with behavioral factors and stress hormone pathogenesis and can increase these hormones. These can increase insulin resistance and create Sympathetic hyperactivity or Sympathetic Excess. Heart Rate Variability (HRV) testing has been used to demonstrate Sympathetic Excess. P&S imbalance can be corrected with normalized ENS activity. The high frequency component of HRV is a measure of Vagal, or Parasympathetic, tone. The ratio of the low frequency HRV component to the high frequency component is an indicator of Sympathovagal Balance (SB, aka, P&S Balance). A meta-analysis has shown that the high frequency or SB components of HRV are affected adversely in a significant proportion of patients with IBS. IBS patients show higher SBs, indicative of a relatively high sympathetic tone at rest as compared with resting Parasympathetic tone. Also, constipation-predominant IBS patients had decreased high frequency activity indicative of low Parasympathetic activity at rest. Other studies of IBS patients have shown abnormalities in cardiac bio-reflex with ANS testing in patients who have autonomic dysregulation.
Therefore, we do recognize abnormalities in the peripheral ANS which can be identified in routine testing in the office setting and balance can be attempted pharmacologically in these areas. However, a recent review [2] describes orthostatic intolerance and postural tachycardia and its association with GI symptoms. The review states that only when the GI symptoms develop in the upright position and then resolve on lying flat can we say that they are causative and related directly to the orthostatic stress. The most common symptoms associated with orthostatic intolerance, include nausea, dyspepsia, bloating and constipation, yet the majority of the subjects do not have gastroparesis. They believe that Postural Orthostatic Tachycardia Syndrome (POTS) is comorbid with many other symptoms such as Migraine Headaches, Fibromyalgia, Chronic Fatigue Syndrome, sleep disorders, abdominal pain, and joint hypermobility (EDS), etc. Further, it is believed that these GI symptoms are also a comorbidity affecting orthostatic intolerance symptoms in POTS patients. They believe POTS is not the driver of the comorbidities, which means that autonomic dysfunction syndromes, such as POTS or orthostatic intolerance symptoms can coexist with GI symptoms but are separate. Although, one cannot discount the common mechanism of brain, heart, blood vessel and brain-gut communications having at times similar mechanisms.
During tilt testing, if gastrointestinal symptoms occur, they usually include nausea and abdominal pain. This suggests that some of these symptoms are related to orthostatic challenges but do not occur universally in all patients. These patients who do have nausea and abdominal pain with tilt usually do respond to volume expanders like fludrocortisone. However, we recognize that treating POTS and orthostatic intolerance with autonomic agents oftentimes does not beneficially affect their GI symptoms if they are not reproduced with tilt test or assuming the upright position (e.g., standing-up). The presence of POTS does not seem to influence the general findings of chronic overlapping pain conditions with functional GI disorders. Our clinical observation, and the empiric observations of others, suggests that a lower vagal modulation may be associated with the more chronic pain disorder rather than what the POTS subjects have in general. Also, these observations suggest that Vasovagal Syncope is more often co-morbid with POTS that previously accepted. Further suggesting Vagal or Parasympathetic dysfunction is involved, including the ENS. Again imbalance in the ENS, Serotonin transmission, and the gut-brain microbiota circuit miscommunications are implicated in IBS.
Therefore, as an autonomic physician, when I see patients with orthostatic intolerance and POTS, even if their GI symptoms may not be directly related, it is important to recognize and understand that the nausea, abdominal pain, constipation, bloating and diarrhea must be managed concomitantly with the P&S dysfunction(s), even though the drug treatments and lifestyle treatments may be different. Interestingly, an altered bowel pattern has been reported in 70% of POTS patients. Diarrhea and constipation, however, are not triggered usually by upright position and tilt. Therefore, they should be treated separately.
In regard to migraines, they are often associated with nausea. This also suggests the involvement of the ANS, specifically the Vagus nerve of the Parasympathetic nervous system. Vagal symptoms are well known in the development of migraine with nausea. The nausea may also be due to autonomic dysfunction in the form of orthostatic challenge, and therefore, one needs to differentiate this. In fact the migraine may also be due to autonomic dysfunction in the form of poor brain perfusion (blood flow to the brain). If the nausea is not relegated to orthostatic challenge and there is no delayed gastric emptying, patients with migraine and nausea can be treated with Cyproheptadine or tricyclic antidepressants or Topiramate.
Dyspepsia is another disorder in which oftentimes a clear-cut mechanism or etiology cannot be found and slow gastric emptying is not present. These patients are oftentimes treated with prokinetic agents like Erythromycin, cholinesterase inhibitors, Buspirone (relaxes the gastric fungus), as well herbal products and tricyclics at low dose, which are better tolerated than SSRIs. One needs, of course, to exclude H. Pylori in these patients. When treating dyspepsia, one starts with H2 blocker or PPPIs to decrease the acid and then can go through the other medicines we have just discussed to see if there is empiric benefit. One must first demonstrate there is a normal upper endoscopy and no H. pylori infection in people who present with dyspepsia. Occasionally, cognitive behavioral therapy and peppermint oil may be used, or occasionally one needs to go to prokinetic agents if tricyclics and SSRIs/SNRIs are not beneficial.
There is a case report of a special treatment of POTS with mast cell activation syndrome using Naltrexone, Immunoglobulins and antibiotic treatment. These authors discussed Intravenous Immune Globulin (IVIG) as an emerging promising therapy for POTS. This is an immunomodulating agent and response to this suggests that some of the autonomic imbalance seen in some POTS patients may be due to active autoimmune muscarinic antibodies against acetylcholine. Many mast cell activation syndrome patients do have GI abnormalities and we should exclude SIBO and IBS in these patients. However, we are not anxious to use immunomodulating agents without more concrete evidence, such as autoantibodies, paraneoplastic antibodies, and so forth, which we can test for. Oftentimes, a consultation with an immunologist and a rheumatologist can be beneficial. There is a link between the autonomics and the immune system, specifically the Parasympathetics control and coordinate the immune system, including Sympathetically mediated histaminergic responses as effected by mast cells.
Vagal, or Parasympathetic, dysfunction with SIBO has been recognized. In a study out of Mt. Sinai Hospital in New York, a subset of patients with chronic inflammation with HIV infection had abnormalities when components of the Vagal nerve were tested. These abnormalities correlated with changes in immune function and GI function in well treated patients with HIV infection. It was postulated that possibly enhancing Vagal function could help benefit HIV patients in the future. The authors emphasized that a function of the peripheral ANS is promotion of GI motility by cholinergic fibers of the Vagus nerve. A potential consequence of Vagal nerve fiber losses slowed motility particularly in the stomach and proximal small intestine with a Vagal nerve context with Enteric neurons. With this slowed motility there is propensity for SIBO. This can promote bacterial translocation, which is what we see in the increased gut permeability syndrome and drive inflammation.
At the other end of the spectrum, cholinergic stimulation, such as provided by the Vagus nerve, has also been shown to modulate GI mucosal inflammation and increase mucosal populations of CD4 cells. CD4 cells are white blood cells that play an important role in the immune system. They provide your body’s natural defense against pathogens, infections and illnesses. CD4 cells are sometimes also called T-cells, T-lymphocytes, or helper cells. Your CD4 cell count gives an indication of the health of your immune system. Independent of the effects on GI motility, Vagal dysfunction could also contribute to chronic inflammation by direct effects on the immune system. These investigators tested patients with sudomotor testing, beat-to-beat blood pressure with tilt and conventional HRV-derived parameters with standard Ewing maneuvers such as Valsalva. They developed a Composite Autonomic Severity Score (CASS) which included sudomotor, Vagal and adrenergic sub-scores. They showed that Vagal dysfunction was associated with slowed gastric emptying and SIBO. They also correlated this with elevated levels of IL-6 and other inflammation markers. They postulated that it is in the GI mechanisms that Vagal dysfunction may be linked to immune dysfunction in HIV patients, and also Vagal dysfunction is linked to chronic inflammation.
Impaired intestinal barrier integrity in the colon of patients with IBS has been demonstrated in prior studies. This involves soluble mediators. These most likely also contributed to chronic inflammation in IBS patients. Again, studies on IBS patients have shown abnormal Vagal dysfunction. Increased GI permeability or “Leaky Gut Syndrome” is a real condition. Simple tests such as Lactulose-Mannitol Intestinal Permeability tests can be done especially when assessing Celiac Disease patients for increased permeability. Other more sophisticated tests such as colonic biopsies looking for mRNA expression in tight junction proteins as has been done with IBS patients to demonstrate increased intestinal permeability is a more elaborate test. There are many tests to assess the intestinal barrier. They have all shown that abnormalities can occur in patients with various GI disorders, such as SIBO and IBS. They have also shown accompanying inflammation and abnormal Vagal tone can be associated with it.
While further research is needed, we strongly advocate in our patients balancing any abnormalities in the general peripheral autonomic (or P&S) nervous system that we encounter especially if there is accompanying orthostatic intolerance or other postural disorders. We advocate exercise, a proper anti-inflammatory diet, and antioxidant supplements along with nitric oxide boost of beetroot. Both stress reduction at the cellular level (known as oxidative stress) and at the whole body level (known as Psychosocial stress) is also a significant part of treating any patients who need to have their P&S nervous systems in balance and who need to have their GI system balanced to function better.
REFERENCES
[1] Mazur M, Furgała A, Jabłoński K, Mach T, and Thor P. Autonomic nervous system activity in constipation-predominant irritable bowel syndrome patients. Med Sci Monit. 2012;18(8):CR493–CR499. doi:10.12659/msm.883269.
[2] Chelimsky G, Chelimsky T. The gastrointestinal symptoms present in patients with postural tachycardia syndrome: A review of the literature and overview of treatment. Auton Neurosci. 2018 Dec;215:70-77. doi: 10.1016/j.autneu.2018.09.003. Epub 2018 Sep 8.



2 Comments
Emma
Thank you very much for your articles,
I am in Australia and I have 8 specialists for hypermobility, Gastroparesis, ibs, POTS and more. Your articles are helping me understand much more clearly what my doctors have been telling me and have given me new insight and understanding.
For the beetroot, do you recommend juice and if so how much?
Thank you
Ranga
I have read both your articles on IBS and orthostatic intolerance. Great articles.
Could antidepressant withdrawal aggravate an underlying condition, for example chronic IBS that is mainly categorized by bloat and burping?
I have noticed since I stopped an antidepressant last year, standing up induces bloat and brain fog, and when I sit down after a long time, it can cause dizziness when I stand up quickly. Also lying down relaxes the gut and there is a lot of gut noise, burping and flatulence – this mainly occurs at night, and I need to be on a low-FODMAP and low-fibre diet. I have also become increasingly developed food intolerance. Last year, I was diagnosed with SIBO, and was treated with anti-microbials, but my SIBO test results were the same, although the symptoms were reduced for 3 months, before I relapsed again in April. However, this time around, I do not have any daytime GI symptoms most days, and it only flares up at night, if I have had trigger foods (high fibre, FODMAP). Interestingly enough, on the days I have had alcohol, the gut remains calm even if I have trigger foods.