Click here to download this post
Notes: This is the third in a series of 5 blog posts about COVID-19 and Autonomic Dysfunction. This a pre-publication release that will be featured in a major medical journal.
Coronavirus Induces Oxidative Stress Leading to Autonomic Dysfunction Often With Delayed Symptom Onset
Heather L. Bloom, MD1 and Joseph Colombo, PhD, DNM, DHS2
- Electrophysiology, Atlanta Veterans Affairs Medical Center and Emory University Medical School, Atlanta, GA bloom@gmail.com
- Parasympathetic & Sympathetic Nervous System Consultant, Franklin Cardiovascular Associates, PA & Autonomic Dysfunction and POTS Center, Sewell, NJ, and Senior Medical Director & CTO, Physio PS, Inc., Atlanta, GA, dovetech@erols.com
Correspondence should be addressed to Dr. Colombo, dovetech@erols.com
COMMON P&S DYSFUNCTIONS CAUSED BY OXIDATIVE STRESS
The ability to simultaneously and (mathematically) independently measure P&S activity under all conditions enables more information and additional abnormal responses [[i]] that have clinical bearing on Dysautonomia symptoms and their therapy. For example a normal postural change or stand response is depicted in Figure 1, Graph A. First the Parasympathetics decrease, potentiating and minimizing the Sympathetic reaction required and then (second) the Sympathetics increase. Lightheadedness due to Dysautonomia is arguably the most debilitating of Dysautonomia symptoms [[ii],[iii]] and results from abnormal stand responses (the rest of Figure 1, and discussed below). Note multiple Dysautonomias may occur simultaneously.
- Challenge Parasympathetic Excess (PE) is an abnormal increase in average Parasympathetic activity during a Sympathetic stimulus (g., stress or exercise), including stand (Figure 1, Graph C). Often the PE forces a secondary, excessive Sympathetic response (Sympathetic Excess or SE) to such stimuli (Figure 1, Graph E). Typically, this is measured as high HR or BP, and treatment responses are often unexpected. Often the HR or BP increases or becomes difficult to manage. This is due to the SE being a secondary response, and possibly compensatory for the underlying Sympathetic Withdrawal (SW) masked by the PE [i]. PE affects brain profusion by effecting circulation throughout the cardiovascular system. Figure 1, Graph D, shows an example of PE with SW (a description of SW is below). [i]
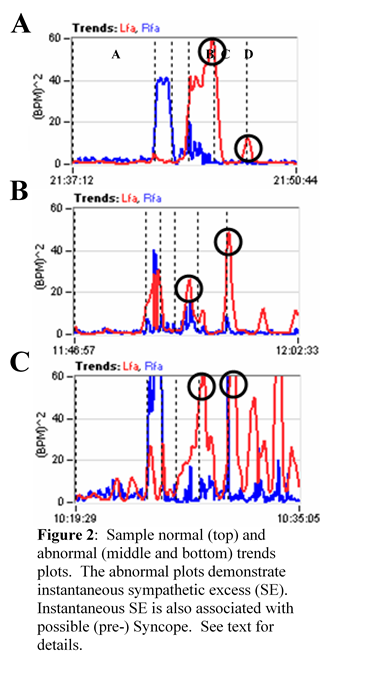
- Head-up postural change (stand) SE (Figure 1, Graph F) is a beta-adrenergic response and is associated with (pre-clinical) Syncope. The Sympathetic response to stand is compared with two other responses: 1) the average resting baseline response and 2) peak (instantaneous) Valsalva response. For the resting response (1), it is well known that the stand Sympathetic response should be higher than at rest, but not too high. The normal range is a 10% to 500% increase over the resting response [[iv],[v],[vi]]. The responses depicted in Figure 1 are average responses over the time period of the stimulus. Sometimes the clinical indications may be averaged out and the instantaneous P&S responses need to be assessed (see Figure 2), such as in comparison with the Valsalva response (2). SE may be documented as a peak Sympathetic response to standing that is comparable to (Figure 2, Graph C), or greater than the peak Sympathetic response to Valsalva (Figure 2, Graph B). Of course this makes no sense, physiologically. The stand Sympathetic response should be significantly lower (< 1/3) than the Sympathetic response to a series of short Valsalva maneuvers (Figure 2, Graph A) which are known to be very significant Sympathetic challenges. (Note: Valsalva maneuvers > 20 seconds are well-known, and significant, Parasympathetic challenges. Valsalva maneuvers < 15 seconds are Sympathetic challenges.) Stand SE is a symptom of poor brain profusion due to insufficient circulation caused by inappropriate autonomic control of the heart (Vasovagal or Neurogenic Syncope) or due to the heart itself (Cardiogenic Syncope). [i]
- Head-up postural change (stand) Sympathetic Withdrawal (SW, Figure 1, Graph B) is an alpha-adrenergic response and is associated with (pre-clinical) orthostatic dysfunction. Any average decrease in Sympathetic activity with standing, as compared with rest is abnormal and considered SW. SW may be accompanied by abnormal BP or abnormal HR responses (g., Orthostatic Hypotension or POTS, respectively). Both PE and stand SE may mask SW. In these cases a weak or abnormal BP response is often still recorded, or treatment of the PE will unmask SW. SW may also present with PE (Figure 1, Graph D). SW affects brain profusion by causing blood volume to shift to the lower extremities, reducing cardiac output and therefore, circulation to the brain. This may lead to hypertension (high systolic BP) as a compensatory mechanism to prevent brain hypoperfusion. It may also be associated with poor cardiac perfusion (low diastolic BP) and, if prolonged, may lead to heart failure. [i]
- Autonomically mediated cardiac arrhythmia (see Figure 3 for an example), including Sinus Arrhythmia, is contra-indicated for heart beat interval analyses, and therefore, contra-indicated for most ANS monitors or measurement devices. With the addition of Respiratory Activity signal analyses to the heart beat interval analyses, more information is available to measure the P&S signals in the “noise” of the arrhythmia. The typical arrhythmia that is associated with P&S dysfunction is Sinus Arrhythmia, which may be described as a normal EKG waveform (a normal heart beat) that occurs with abnormal timing (due to an abnormal P or S input to the heart). As a result, autonomically mediated cardiac arrhythmia may be perceived as “skipped-beats” or “rapid-beats” or, in general, palpitations.
FIGURES and FIGURE LEGENDS

REFERENCES
_________________
[1] Murray GL. COVID-19 cardiac complications: Is an easy, safe treatment strategy right under our noses? J Cardiovasc Dis Diag. 2020; 8:5. doi: 10.37421/jcdd.2020.8.415.
2 DePace NL, Colombo J. Autonomic and Mitochondrial Dysfunction in Clinical Diseases: Diagnostic, Prevention, and Therapy. Springer Science + Business Media, New York, NY, 2019.
3 Acosta C, DePace NL, DePace NL, Kaczmarski K, Pinales JM, and Colombo J. Antioxidants effect changes in systemic parasympathetic and sympathetic nervous system responses and improve outcomes. Cardio Open. 2020; 5(1): 26-36. doi: 10.33140/COA.05.01.04
4 Colombo J, Arora RR, DePace NL, Vinik AI. Clinical Autonomic Dysfunction: Measurement, Indications, Therapies, and Outcomes. Springer Science + Business Media, New York, NY, 2014.
5 Vinik A, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007; 115: 387-397.
6 Vinik AI, Maser RE, Nakave AA. Diabetic cardiovascular autonomic nerve dysfunction. US Endocrine Disease. 2007; Dec: 2-9.
7 Malik, M. The Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability, standards of measurement, physiological interpretation, and clinical use. Circulation. 1996; 93:1043-1065.
8 Malik, M. and the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability, standards of measurement, physiological interpretation, and clinical use. European Heart Journal. 1996, 17: 354-381.
9 Akselrod S, Oz O, Greenberg M, Keselbrener L. Autonomic response to change of posture among normal and mild-hypertensive adults: investigation by time-dependent spectral analysis. J Auton Nerv Syst. 1997 May 12;64(1):33-43.
10 Piña IL, Di Palo KE, Ventura HO. Psychopharmacology and Cardiovascular Disease. JACC. 2018; 71(20): 2346-2359.
11 Arora RR, Bulgarelli RJ, Ghosh-Dastidar S, Colombo J. Autonomic mechanisms and therapeutic implications of postural diabetic cardiovascular abnormalities. J Diabetes Science and Technology. 2008; 2(4): 568-71.
12 DePace NL, Vinik AI, Acosta C and Colombo J. Oral vasoactive medications: A Review of Midodrine, Droxidopa, and Pseudoephedrine as Applied to Orthostatic Dysfunction. NEJM. 2020. Submitted.
13 Vinik AI, Bloom HL, Colombo J. Differential effects of adrenergic antagonists (carvedilol vs. metoprolol) on parasympathetic and sympathetic activity: A comparison of measures. Heart International. Heart Int. 2014; 9(1): 7-14; DOI: 10.5301/HEART.2014.12495.
14 Bloom HL, Vinik AI, Colombo J. Differential effects of adrenergic antagonists (carvedilol vs. metoprolol) on parasympathetic and sympathetic activity: A comparison of clinical results. Heart Int. 2014 ; 9 (1): 15-21; DOI: 10.5301/HEART.2014.12496.
15 Murray GL and Colombo J. (R)Alpha Lipoic Acid is a Safe, Effective Pharmacologic Therapy of Chronic Orthostatic Hypotension Associated with Low Sympathetic Tone. Int J Angiol. In Print, 2018.
KEY WORDS
Coronavirus, Parasympathetic, Sympathetic, Oxidative Stress, Antioxidants
ABBREVIATIONS
ALA Alpha-Lipoic Acid
ANS Autonomic Nervous System
CoQ10 Co-enzyme Q10
COVID-19 Coronavirus (SARS-CoV-2)
P&S Parasympathetic and Sympathetic
PE Parasympathetic Excess
POTS Postural Orthostatic Tachycardia Syndrome
SE Sympathetic Excess
SW Sympathetic Withdrawal
[i] Colombo J, Arora RR, DePace NL, Vinik AI. Clinical Autonomic Dysfunction: Measurement, Indications, Therapies, and Outcomes. Springer Science + Business Media, New York, NY, 2014.
[ii] Vinik A, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007; 115: 387-397.
[iii] Vinik AI, Maser RE, Nakave AA. Diabetic cardiovascular autonomic nerve dysfunction. US Endocrine Disease. 2007; Dec: 2-9.
[iv] Malik, M. The Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability, standards of measurement, physiological interpretation, and clinical use. Circulation. 1996; 93:1043-1065.
[v] Malik, M. and the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability, standards of measurement, physiological interpretation, and clinical use. European Heart Journal. 1996, 17: 354-381.
[vi] Akselrod S, Oz O, Greenberg M, Keselbrener L. Autonomic response to change of posture among normal and mild-hypertensive adults: investigation by time-dependent spectral analysis. J Auton Nerv Syst. 1997 May 12;64(1):33-43.



