Click here to download this post
Patients with Postural Orthostatic Tachycardia Syndrome (POTS) are often quite symptomatic and have Orthostatic Intolerance (an abnormal blood pressure in response to upright posture, including standing) and Orthostatic Tachycardia (a high heart rate response to standing). Many times, there is an antecedent viral infection and this suggests that there may be an element of autoimmunity triggered by a viral infection. Note, tachycardia is mediated by beta-Adrenergic nerves innervating the heart and orthostatic dysfunction is due to an alpha-Adrenergic insufficiency in the lower vasculature. POTS patients may also demonstrate a Parasympathetic Excess, further exacerbating their condition. Due to the fact that all three of these disorders involve three different portions of the autonomic nervous system, all three dysautonomias may present simultaneously.
Orthostatic dysfunction is one form of autonomic dysfunction. It is of the earliest results of autonomic dysfunction and perhaps the most debilitating symptom of autonomic neuropathy. Orthostatic Hypotension is one form of Orthostatic Intolerance. Orthostatic Hypotension presents as a significantly abnormal drop in blood pressure in response to upright posture, including standing or head-up tilt table test. In fact any blood pressure response to standing that is less than a 10 mmHg increase in systolic blood pressure upon standing is considered abnormal. Specifically, Orthostatic Hypotension is defined as a decrease in blood pressure upon standing of more than 20/10 mmHg pressure, and other changes of less than a 10 mmHg increase in systolic blood pressure upon standing is considered to be Orthostatic Intolerance. Other autonomic forms of Orthostatic Dysfunction include Postural Orthostatic Tachycardia Syndrome and, rarely, Orthostatic Hypertension (an excessive increase in blood pressure upon standing). While there are several underlying reasons for Orthostatic Dysfunction, other than autonomic dysfunction (e.g., venous valve dysfunction and dysfunction of the smooth muscles in the walls of the lower vasculature), the underlying autonomic dysfunction is known as Sympathetic Withdrawal.
Normally, upon standing, the Parasympathetics first decrease to potentiate and minimize the (alpha-) Sympathetic response. The Parasympathetic 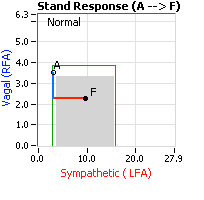 decrease is represented by the blue line decreasing, going down, in the figure to the right. This begins the process of vasoconstriction to move blood up to the abdomen to help the heart pump blood to the brain. Then the Sympathetics increase (represented by the red line increasing, going to the right, in the figure to the right). This Sympathetic increase sustains the vasoconstriction and continues to shift the majority of the blood volume from the feet, against gravity, to the abdomen so that the heart may more easily pump it to the brain.
decrease is represented by the blue line decreasing, going down, in the figure to the right. This begins the process of vasoconstriction to move blood up to the abdomen to help the heart pump blood to the brain. Then the Sympathetics increase (represented by the red line increasing, going to the right, in the figure to the right). This Sympathetic increase sustains the vasoconstriction and continues to shift the majority of the blood volume from the feet, against gravity, to the abdomen so that the heart may more easily pump it to the brain.
Think of a car as the model. The Parasympathetics are the brakes and the Sympathetics are the accelerator. When stopped at a red light with your foot on the brakes and the light turns green, what is the first thing you do? … You take your foot off the brakes. Even before you touch the accelerator, you begin to roll, you already begin to accelerate. Taking your foot off the brakes minimizes the amount gas (read that as Adrenaline) and 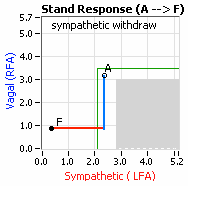 acceleration (read that as Sympathetic stress) you need to reach your desired speed. The Parasympathetic and Sympathetic nervous systems normally act in much the same manner: first the Parasympathetics decrease to facilitate and minimize the Sympathetic response, and then the Sympathetics increase. Sympathetic Withdrawal is the abnormal decrease in alpha-Sympathetic activity upon standing (see figure, left).
acceleration (read that as Sympathetic stress) you need to reach your desired speed. The Parasympathetic and Sympathetic nervous systems normally act in much the same manner: first the Parasympathetics decrease to facilitate and minimize the Sympathetic response, and then the Sympathetics increase. Sympathetic Withdrawal is the abnormal decrease in alpha-Sympathetic activity upon standing (see figure, left).

Note, women tend towards Postural Orthostatic Tachycardia Syndrome (POTS). This is due to the fact that, on average, women are born with physically smaller hearts than men. Therefore, when their hearts become deconditioned, their hearts do not have the leverage to increase pressure to deliver more blood to the brain, so it resorts to the only other way and that is to increase rate to deliver more blood to the brain. This increased rate is Tachycardia, see figure, right: the upper panel displays the Sympathetic Withdrawal and the lower panel displays the instantaneous respiratory (gray trace) and heart rate (red trace) during the first five-minutes of standing from a seated posture. Note how the heart rate does not return to baseline as would be normal, but increases and continues to increase throughout the stand period and, for the most part, exceeds 120 bpm.
In all patients with Orthostatic Dysfunction, a deconditioned heart is a primary disorder. A deconditioned heart does not necessarily mean that the skeletal muscles of the body are deconditioned. Patients with Orthostatic Dysfunction and deconditioned hearts are often in good physical condition and are (or were) able to exercise, even rigorously. In fact the exercise made them feel better (temporarily) because it used the skeletal muscles to help bring blood to the heart to improve circulation. Their feet were warmer and in less pain and their brains were better perfused and more “awake,” less “brain-fog” and memory or cognitive difficulties. The exercise was a form of temporary, self-medication. While exercise is ultimately the best medicine to re-condition the heart, the alpha-Sympathetic nerves also need to be “retrained” to respond properly and increase to cause the required vasoconstriction needed to support the heart. Often this exercise needs to be low and slow, so as to not over-stress the nervous system. A standard to consider is 40 minutes of exercise per day, walking at no more than 2 mph, every day for six months.
On another note, Autonomic Dysfunction may involve multiple dysfunctions. Often, Orthostatic Dysfunction (Sympathetic Withdrawal) may be 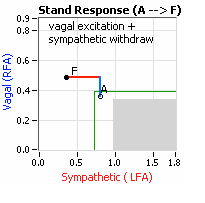 accompanied by a Vagal or Parasympathetic Excess (see figure, right). Parasympathetic Excess may be associated with Vasovagal Syncope. The Parasympathetic Excess (represented by the blue line increasing in the figure, right) is the Vagal component, followed by the Sympathetic Withdrawal. With Parasympathetic and Sympathetic Monitoring (P&S Monitoring, aka, Cardiorespiratory Monitoring) separate, but simultaneous measurements of Parasympathetic and Sympathetic nervous system activity is available in an easy to administer and perform test in the clinic. With documentation of both Sympathetic Withdrawal and Parasympathetic Excess, both conditions may be treated simultaneously: one treatment to reverse Sympathetic Withdrawal (e.g., Midodrine, Mestinon, or Alpha-Lipoic Acid) and one treatment to relieve Parasympathetic Excess (e.g., very, low-dose Anticholinergics or low and slow Exercise).
accompanied by a Vagal or Parasympathetic Excess (see figure, right). Parasympathetic Excess may be associated with Vasovagal Syncope. The Parasympathetic Excess (represented by the blue line increasing in the figure, right) is the Vagal component, followed by the Sympathetic Withdrawal. With Parasympathetic and Sympathetic Monitoring (P&S Monitoring, aka, Cardiorespiratory Monitoring) separate, but simultaneous measurements of Parasympathetic and Sympathetic nervous system activity is available in an easy to administer and perform test in the clinic. With documentation of both Sympathetic Withdrawal and Parasympathetic Excess, both conditions may be treated simultaneously: one treatment to reverse Sympathetic Withdrawal (e.g., Midodrine, Mestinon, or Alpha-Lipoic Acid) and one treatment to relieve Parasympathetic Excess (e.g., very, low-dose Anticholinergics or low and slow Exercise).
In an article by Li and coworkers in the Journal of American Heart Association in 2014, the authors showed that patients with POTS have elevated levels of Alpha 1 AR autoantibodies. These exert a partial peripheral antagonist effect which causes a compensatory Sympathetic activation of the Alpha 1 AR for vasoconstrictors and the Beta AR-mediated tachycardia. They concluded that coexisting Beta 1 AR and Beta 2 AR agonist autoantibodies facilitated a tachycardia. They felt that this may explain the increased standing plasma norepinephrine and excessive tachycardia observed in many POTS patients, the so called hyperAdrenergic POTS syndrome. They examined the serum of 14 POTS patients and concluded that the POTS serum acted as a partial Alpha-1 antagonist and caused a compensatory Sympathetic activation. They concluded that their data supported an autoimmune mechanism for POTS patients. Perhaps future management, they predicted, would ideally block autoantibody activity and leave the receptors unblocked.
In a diagram in the article, they show how in the upright position of POTS patients there is pooling of blood in the veins and a slight drop in blood pressure, which causes a baroreceptor activation. Alpha 1 AR-Ab impaired vasoconstriction results, and this is an impaired Alpha 1 AR-mediated vasoconstriction. This increases the drop of blood pressure, which causes an exaggerated baroreceptor activation then an exaggerated sympathoneural response with resultant tachycardia. These investigators also found that Beta 1 AR activating autoantibodies were also present in all of their POTS patients tested, and this facilitated the Beta 1 AR agonist activation in in vitro testing with cyclic AMP. There was also a variable presence of Beta 2 AR autoantibodies. These autoantibodies contribute to exaggerated tachycardia in POTS patients also. They postulated that these antibody receptors may also cause abnormal neurohumoral responses in people with cardiomyopathies.
In the Annals of Clinical Translational Neurology, an article published by Watari and co-workers, evaluated the association between POTS and circulating anti-ganglionic Acetylcholine receptors (gACHR) antibodies. They used a special test for gACHR antibodies, known as the Luciferase Immunoprecipitation System. These investigators found that antecedent infections were common in POTS patients. They also had autoimmune markers and comorbid autoimmune diseases frequently in seropositive POTS patients. Anti-gACHR antibodies were present in a significant number of POTS patients. They had two groups of patients. Ten were seropositive for autoantibodies with POTS and ten POTS patients who were seronegative. They found that antibodies were more frequently detected in patients with POTS than patients with neurally mediated syncope (NMS). This was an observational study, but it showed that anti-gACHR were detected more frequently in patients with POTS compared to vagal syncope patients. This supported an autoimmune mechanism for at least 29% of POTS patients who had anti-gACHR Alpha 3 and Beta 4 antibodies in the serum from POTS patients. In 2016, Fedorowski demonstrated a strong relationship between Adrenergic antibodies in patients with POTS. They showed the shift in Alpha 1 AR and Beta 1 AR responsiveness is important in the pathophysiology of POTS. A large percentage of the POTS patients had autoantibodies that activated Alpha 1 AR, Beta 1 AR and Beta 2 AR, respectively.
They concluded that their studies affirmed the concept that common cardiovascular dysautonomias includes a spectrum of autoantibodies which contributed to the clinical manifestation. They compared this with inappropriate sinus tachycardia (IST) with circulating antibodies against cardiac B receptors, as previously reported by Chiale (Heart Rhythm, 2006). They emphasized that the catecholamine surge in POTS patients is seen as a compensatory mechanism to override the Alpha 1 AR malfunction with autoimmune blockade seen in POTS patients, but not seen in vagal syncope patients.
An article by Gunning and his coworkers in the Journal of the American Heart Association, volume 8, #18, discussed POTS associated with elevated G-protein coupled receptor autoantibodies. The authors noted that in most cases the POTS patients had at least one elevated G-Protein coupled Adrenergic autoantibody, and in some instances, both Adrenergic and Muscarinic autoantibodies which supports the hypothesis that POTS may be an autoimmune mechanism disorder. They evaluated antibodies levels against four subtypes of G-Protein coupled Adrenergic receptors and five subtypes of G-Protein coupled Muscarinic Acetylcholine receptors by an ELISA technique. Eighty-nine percent of patients had antibodies against the Adrenergic Alpha 1 receptor, and 53 percent against the Muscarinic Acetylcholine M4 receptor. Four patients had elevations of G-Protein coupled antibodies against all nine receptor subtypes measured in their study. Five POTS patients had no elevation of any autoantibody and controls had no elevation. They postulated that their findings suggested that possibly immunomodulating medications may be a therapeutic target in the future for POTS patients who are refractory to other forms of treatment.
POTS affects 3 million people in the United States, particularly young women of childbearing age. Many mechanisms related to the etiology of POTS demonstrate that viral infections, Celiac disease, Thyroiditis, and joint Hypermobility may trigger it. The authors used ELISA kits purchased from CellTrend GmbH (Luckenwalde-Germany) to detect antibodies against nine different G-Protein coupled receptor antibodies, including four anti-human AdrR epitopes and five anti-human mAChR epitopes. The authors cited Li reporting antibodies to Beta Adrenergic B2 and Muscarinic M3 receptors by ELISA and 75% of patients with significant Orthostatic Hypotension and that subsequently antibodies of both Adrenergic Alpha 1 and Beta 1 receptors were reported in POTS patients along with angiotensin 2-type autoantibodies also found in POTS patients. The most prevalent autoantibody in their investigations was anti-Adrenergic A1 receptor and that one had to have an elevation of autoantibodies against A1 to also have other Adrenergic and Muscarinic receptor autoantibodies. The A1 Adrenergic receptor function is a vasoconstriction and antibodies specific to this G-Protein coupled protein receptor would therefore cause an ineffective response to simulating resultant hypotension and then a compensatory tachycardia would result through a baroreflex mechanism.
In the Journal of American Heart Association recently, an article titled Adrenergic Aorta Antibody-Induced Postural Tachycardia Syndrome in Rabbits, Li and coworkers build on their previous work of Adrenergic autoantibody in POTS. In this study, they develop and Adrenergic receptor peptide-immunized rabbit model. The Adrenergic antibodies were similar to antibodies isolated from patients with POTS syndrome. The POTS-like phenotype in rabbits was induced by these Adrenergic autoantibodies, and the rabbits actually demonstrated postural orthostatic tachycardia. This study showed that there is an animal model of POTS based on autoimmune causes. The immunization of rabbits with Adrenergic receptor peptides induced a POTS-like presence of symptoms and orthostatic tachycardia. In an article by Miller and Doherty entitled Hop To It: The First Animal Model of Autoimmune Postural Orthostatic Tachycardia Syndrome, they review the importance of the work done by Dr. Li with the rabbit model. They also were impressed by not only the Adrenergic autoantibodies inducing a POTS-like phenotype in rabbits which exacerbated orthostatic tachycardia and produced Adrenergic receptor dysfunction, but this was suppressed by selectively clearing the antibodies in vivo. This gives promise to future research in humans if an autoimmune mechanism could be further substantiated.
Autoantibodies to Adrenergic receptors contribute to the pathophysiology of POTS is a hypothesis. The Adrenergic receptors are key regulators of blood pressure and heart rate. Patients with POTS have impaired Alpha 1 Adrenergic receptor 1-induced vasoconstriction and compensatory enhanced Beta 1 Adrenergic receptor-induced tachycardia.
The study by Li on rabbits not only develops an animal model but a potential target of therapy for POTS. It established a target immune therapy, the potential therapy for POTS. Twenty-five percent of POTS patients become disabled and cannot work or attend school. In the United States, for immune therapy targeting autoantibodies, we rely on plasma exchange, Intravenous Immunoglobulins (IVIG), and possibly B cell depleting strategies. There are risks with plasma exchange, however, including hypotension, coagulopathy, central access problems, etc. IVIG has adverse effects, including inflammatory reactions, Hemolytic Anemia and Aseptic Meningitis. B cell depleting therapy such as with Rituximab, which targets CD20+ B cells to remove B cell populations that are precursors to antibodies producing plasma cells, has been considered. However, this agent has a strong safety profile although infections and severe reactions to first infusions can occur. There are other new techniques being developed for immunoabsorption that could be promising in the future. The question remains, will the rabbit model of POTS be representative of the various presentations of the patient’s population with POTS and further research needs to be done. In an article by Gunning, coworkers and Grubb, they note that POTS is usually misdiagnosed as chronic anxiety or panic disorder because their autonomic failure is not usually severe. However, they nicely demonstrated 89% Adrenergic Alpha 1 receptor antibodies in 53% Muscarinic Acetylcholine antibodies in the patient patients with POTS.
All of this data points to an autoimmune mechanism in POTS as possibly the common final pathway. An animal model now produced may be very useful in research.
Also, Yu and coworkers in the Journal of American Heart Association published an article, Angiotensin II Type 1 Receptor Autoantibodies in Postural Orthostatic Tachycardia. They acknowledge that autoantibodies to Alpha 1-Adrenergic and Beta 1/2-Adrenergic receptors had previously been found in serum from patients with POTS. They investigated the role of AT1R autoantibodies in POTS patients. They found that most patients with POTS did have AT1R antibody activity. This supported the concept that AT1R autoantibodies and anti-Adrenergic autoantibodies act separately or together and exert a significant impact on the cardiovascular pathophysiology characteristic of POTS.
In total, all of this data shows that with POTS patients there is an association with autoantibodies to various Adrenergic receptors and Angiotensin receptors. The animal model makes a cause and effect theory plausible to fulfil Hills Criteria of Causation.
Unfortunately, testing for antibodies to these receptors is still in the experimental stage and no definitive treatment has been published in controlled studies. However, this is very promising research information for future endeavors.




2 Comments
Alice Carroll
It’s helpful to know that there is a POTS syndrome treatment that can be paired with slow exercises. My father is thinking about looking for a POTS syndrome doctor because his checkup recently revealed that he might be susceptible to it. Speaking with an expert would surely make things clearer.
GG
Thank you for posting this. It helped me a lot.