Click here to download this post
Dr. DePace, MD, FACC
Symptoms of Chronic Fatigue Syndrome
Chronic diseases usually last for over 6 months. In Chronic Fatigue, we see post-exertional fatigue, unrefreshing sleep, and “Brain Fog” ( memory and cognitive disturbances).
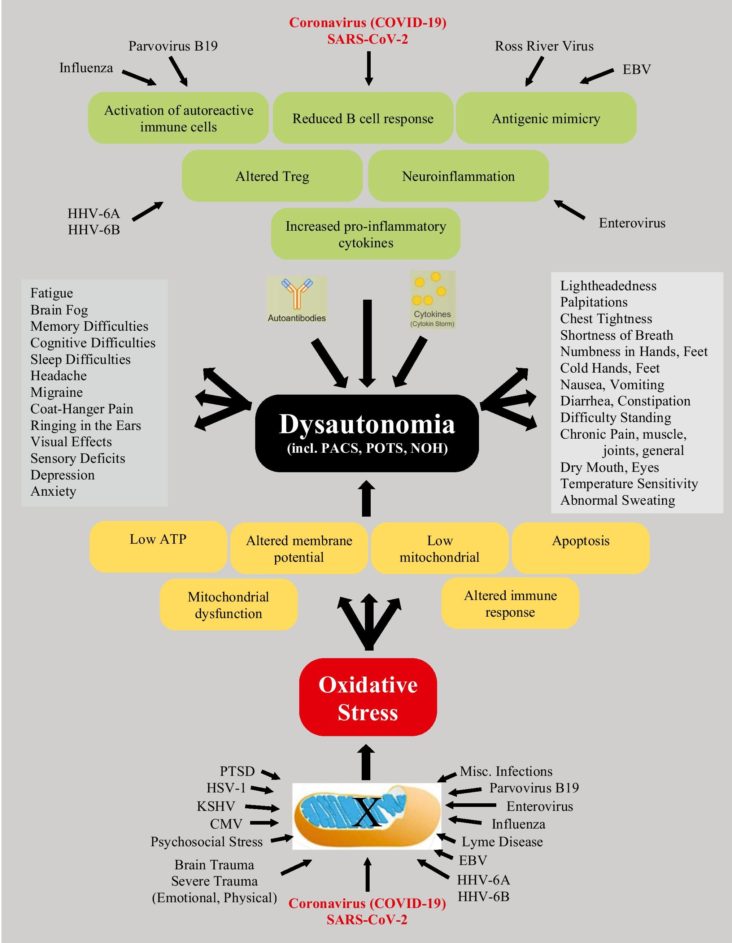
The Autonomic Nervous System (Parasympathetic and Sympathetic balance) is often abnormal in Chronic Fatigue Syndrome (CFS). This affects blood pressure (BP) and heart rate (HR) regulation.
That’s why we see Orthostatic Intolerance in most cases like Postural Orthostatic Tachycardia Syndrome (POTS). In this condition they experience worsening symptoms when are in an upright position and improve when they lie down. Females are more affected than males. As many as 8 million Americans may be affected. Patients are affected at different ages and have different presentations.
What Causes Chronic Fatigue Syndrome?
Chronic Fatigue Syndrome is a group of disorders that consists of many different causes. Let’s take them separately;
- Infection (viral or bacterial) that causes autoantibodies and oxidative stress to dysregulate cellular and specifically mitochondrial energetics. This may lead to exercise intolerance.
- Disturbed gut microbiota (abnormal bacterial colonization) possibly leading to “leaky-gut” leads to autoimmunity. Irritable Bowel Syndrome is seen in many Chronic Fatigue patients.
- Microglial activation of the nervous system, including the Central nervous System (CNS), possibly leading to chronic pain due to allodynia (pain due to stimuli that is usually not painful) and hyperalgesia (abnormally heightened sensitivity to pain).
- Neuronal inflammation is important in the pathophysiology of many disabling symptoms.
- High levels of pro inflammatory cytokines (chemicals produced by cells) and low level of antioxidants, such as Coenzyme Q-10 (CoQ10) or Glutathione
- Abnormalities of the Hypothalamus-Pituitary-Adrenal Axis possibly leading to “delayed cortisol awakening”, possibly leading to unrefreshing sleep. In some cases we see low cortisol levels. Cortisol is a hormone that helps the body handle stress.
- Physical or emotional trauma, including form an accident, concussion, immobilization, surgery, trauma, or even emotional stress such as loss of a loved one.
- Genetics may contribute, with identical twins having a higher incidence then fraternal twins. There has also been familial aggregations note of CFS.
- Environmental factors like mold or toxins may also be a triggers.
While mitochondrial dysfunction is implicated as an immediate cause of CFS, it is not determined what the damage to mitochondrial function is from.
Mitochondria are components of cells that are called organelles and they produce energy in the form of a molecule called ATP. Cellular hypoxia and oxidative stress happen during stressful situations.
Treatments For Chronic Fatigue Syndrome
The end result is disturbing muscle and nerve function. Exercise is the hallmark treatment for improving CFS. “Low and slow” exercise is where patients exercise 2-5 minutes followed by 5 minutes of rest so as not to damage skeletal muscle. Another such exercise is walking slowly, no more than 2 MPH for 40 minutes daily.
Even if biking or rowing, no more than 2 MPH. This may be too stressful for some patients, who on some days cannot lift their heads off the pillow. Supine exercises can be used for them. More work is required to assess the types of exercise programs that are most effective.
Diets high in processed foods and full of chemicals may be a cause of CFS and should be avoided. Cocktails of antioxidants that work on the mitochondria and immune system modulation are current areas of investigation. Currently, we are working on ways to categorize the different patients to determine which treatments work best.