Click here to download this post
Dr. Nicholas L. DePace, M.D., F.A.C.C.
ME/CFS is a common and very debilitating disease for which the origin, or etiology, is unknown. While there is some controversy about the exact cause or causes, much has been learned in the last 20 years. One widely held theory is that patients with a genetic predisposition and abnormal bacteria colonization, or dysbiosis, experience a gradual development of lymphocytes which are known as B cell clones which are susceptible to autoreactivity. Normally these B cells produce normal antibodies in the body. However, during unusual circumstances a triggering event such as a viral or a bacterial infection can cause these B cells to become autoreactive and produce autoantibodies. Therefore, there was some belief there may be an autoimmune mechanism which begins evolving and causes this disease process.
ME/CFS is a chronic disease that usually has lasted for more than six months. The result is post-exertional fatigue, unrefreshing sleep, memory and cognitive disturbances (“Brain Fog”), and oftentimes Autonomic Nervous System dysfunction (typically involving Parasympathetic Excess, an abnormal increase in Parasympathetic activity in response to a Sympathetic challenge or stress). Usually the stricken individual was very active prior to the onset of the disease. The disease usually persists as a chronic condition. Females are affected more than males. As many as 8 million Americans may be affected. While the cause of ME/CFS is unknown, many factors are through to contribute to the development of the illness, such as: (1) bacterial or viral infections, or (2) physical or emotional trauma, including from an accident, concussion, immobilization, surgery, trauma, or even a significant emotional stress such as loss of a loved one. Genetics may also contribute, and a genetic link with common environmental exposures, such as infectious or toxic has been postulated. Identical twins have a higher incidence then fraternal twins. Environmental factors, such as molds or toxins may also be a trigger to ME/CFS. However, no one common cause has been identified. This is because the population is heterogenous. Patients are affected at different ages and have different presentations.
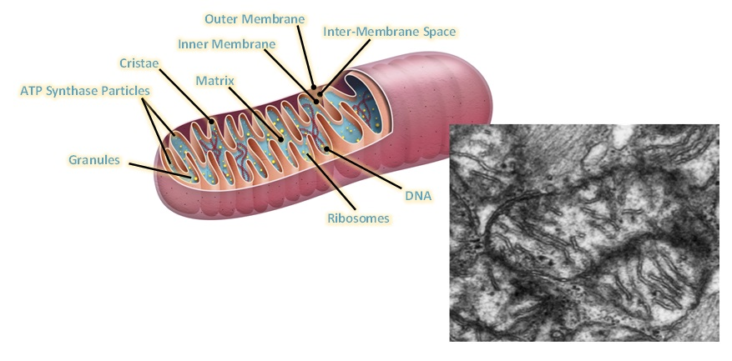
Dysfunctional energetics at the cellular level is believed to be a common mechanism. Disturbed muscle function, metabolism, mitochondrial function, immunity, signaling, neurological, and adrenal and gut health are involved. Specifically, abnormal metabolism regarding the mitochondria has been  demonstrated. Urea Cycle dysregulation, Tricyclic Carboxylic Acid (TCA) Cycle disturbances, and dysregulation of Amino Acid metabolism are also involved. Also, gut microbiota disturbances have been identified. In regard to Mitochondrial dysfunction, studies state that ATP8 levels have been both noted to be reduced and elevated, and resting ATP8 synthesis rates have been variable. However, studies on isolated Peripheral Blood Mononuclear Cells have shown that under stress such as Hypoglycemia there is inefficient ATP8 production in Chronic Fatigue patients but not in normal controls. This was demonstrated by Tomas and coworkers in 2017. Therefore, while resting ATP studies show that production may not be significantly abnormal in ME/CFS patients as compared with controls, it appears that under stressful situations, such as Hypoglycemia, the situation is different when one analyzes peripheral blood mononuclear cell ATP production. ATP is the energy molecule of the cell and of the body and is produced in the Mitochondria, which are the energy factories of the body.
demonstrated. Urea Cycle dysregulation, Tricyclic Carboxylic Acid (TCA) Cycle disturbances, and dysregulation of Amino Acid metabolism are also involved. Also, gut microbiota disturbances have been identified. In regard to Mitochondrial dysfunction, studies state that ATP8 levels have been both noted to be reduced and elevated, and resting ATP8 synthesis rates have been variable. However, studies on isolated Peripheral Blood Mononuclear Cells have shown that under stress such as Hypoglycemia there is inefficient ATP8 production in Chronic Fatigue patients but not in normal controls. This was demonstrated by Tomas and coworkers in 2017. Therefore, while resting ATP studies show that production may not be significantly abnormal in ME/CFS patients as compared with controls, it appears that under stressful situations, such as Hypoglycemia, the situation is different when one analyzes peripheral blood mononuclear cell ATP production. ATP is the energy molecule of the cell and of the body and is produced in the Mitochondria, which are the energy factories of the body.
Mitochondria are organelles, or components of cells, which are very active and contain their own DNA contents separate from the nucleus of the cell. Elevated oxidative stress has also been demonstrated in many subpopulations of patients with ME/CFS. Increasing oxidative stress has been demonstrated with testing products which are the result of oxidative stress, which include increased isoprostane, increased oxidized LDL levels, and increased iso-prostaglandin F2 levels. Also, reduced protective antioxidants, such as glutathione levels have been reduced in populations of patients with ME/CFS. Oxidative stress is produced when free radicals are produced in the mitochondria of cells in abundance during stressful situation and in essence cause a chemical burning reaction in damaged tissues.
 Figure Legend: Schematic diagram showing various viral pathogens potentially associated with ME/CFS and possible molecular mechanisms altered by these pathogens that can contribute to ME/CFS development [[i]].
Figure Legend: Schematic diagram showing various viral pathogens potentially associated with ME/CFS and possible molecular mechanisms altered by these pathogens that can contribute to ME/CFS development [[i]].
Plioplys and coworkers demonstrated lower levels of serum total Carnitine, free Carnitine, and Acetylcarnitine compared to healthy controls, and the lower level correlated with the more severe disease and ME/CFS patients. Carnitine is an important natural component in transporting Fatty Acids across the Mitochondria cell membrane to continue the process of fatty acid oxidation, which also produces ATP molecules.
In regard to ATP molecules, Mayhill and coworkers measured Mitochondria function and ATP production in Neutrophils and developed an ATP profile test. More elements of the ATP profile are abnormal in patients with ME/CFS. Again, this reinforces the fact that there are abnormal energetics occurring within the Mitochondria of cells. They state “our observations strongly implicate Mitochondrial dysfunction as the immediate cause of chronic fatigue symptoms. However, we cannot tell whether the damage to Mitochondria function is a primary effect or a secondary effect to one or more of a number of comorbidities, for example, cellular hypoxia or oxidative stress, including excessive peroxynitrates.”
[i] Rasa S, Nora-Krukle Z, Henning N, Eliassen E, Shikova E, Harrer T, Scheibenbogen C, Murovska M, and Prusty BK. Chronic viral infections in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J Translational Med. 2018; 16: 268, doi:10.1186/s12967-018-1644-y.
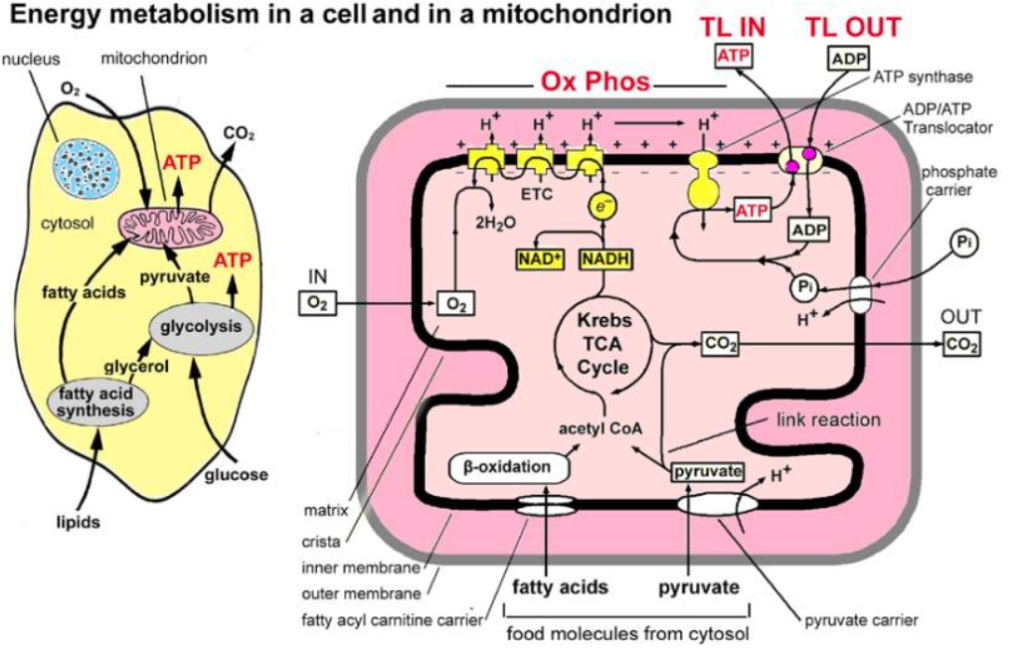
Figure Legend: Main stages and location of energy metabolism in a human cell (left), and simplified details of a mitochondrion showing the main metabolic cycles and the oxidative phosphorylation respiratory chain (right). The outer mitochondrial membrane is highly permeable whereas the inner membrane is permeable only to water and gases. Special carrier and Translocator proteins pass reactants through it. At the top are the proteins involved in the respiratory electron transfer chain (ETC) and in the transfer of ATP and ADP between the cytosol and mitochondrion. ADP and Pi are combined by ATP synthase to make ATP. The ADP/ATP Translocator opens OUT to transfer ADP into the matrix and opens IN to transfer ATP to the cytosol. Nicotinamide adenine dinucleotide plays a key role in its oxidized form NAD+ and its reduced form NADH + H+ in carrying and transferring protons (H+) and electrons (e−) [[i]].
Key reports on ME/CFS have shown abnormal metabolites produced which demonstrate disturbed Amino Acid metabolism, dysregulated lipid metabolism with possible glycolysis impairment, possible Pyruvate Dehydrogenase (PDH) impairment, Urea Cycle dysregulation and overall TCA cycle substrates provision deficiency and reliance these cells for alternate fuel sources. As noted, Mitochondria function has been shown to be abnormal and the Electron Transport Chain, specifically if Complex IV is inefficiently compensated for the up-regulation of supporting pathways.
[i] Myhill S, Booth NE, McLaren-Howard J. Chronic fatigue syndrome and mitochondrial dysfunction. Int J Clin Exp Med. 2009; 2(1): 1–16.

Abnormalities in B cells have been linked to mitochondrial disturbances and as gut microbiota and physiology. Autoimmunity has been little  researched but has been performed on a subtype that is especially comorbid with Irritable Bowel Syndrome, which is seen in many Chronic Fatigue patients. Autoimmune evidence has been strengthened by the fact that there is a decrease in the natural killer cell cytotoxicity in patients with ME/CFS. Natural killer cells are Granular Lymphocytes which attack viruses and bacteria foreign to the body. In addition, the autoimmune evidence is supported by autoantibodies which have been noted against various transmitter receptors, both Muscarinic receptors and Beta receptors. A high incidence of these receptors has also been found in patients with Postural Orthostatic Tachycardia. Specifically, autoantibodies against the Muscarinic and Cholinergic receptors #3 (M3) and autoantibodies against the Muscarinic and Cholinergic receptor #4 M4) are elevated in 20-30% of all patients suffering from ME/CFS. Other studies have shown Beta-1 Adrenergic Receptor Autoantibodies and Beta-2 Adrenergic Receptor Autoantibodies along with Alpha-1 Adrenergic Receptor Autoantibodies, the same autoantibodies which we find in a significant number of patients with Postural Orthostatic Tachycardia Syndrome.
researched but has been performed on a subtype that is especially comorbid with Irritable Bowel Syndrome, which is seen in many Chronic Fatigue patients. Autoimmune evidence has been strengthened by the fact that there is a decrease in the natural killer cell cytotoxicity in patients with ME/CFS. Natural killer cells are Granular Lymphocytes which attack viruses and bacteria foreign to the body. In addition, the autoimmune evidence is supported by autoantibodies which have been noted against various transmitter receptors, both Muscarinic receptors and Beta receptors. A high incidence of these receptors has also been found in patients with Postural Orthostatic Tachycardia. Specifically, autoantibodies against the Muscarinic and Cholinergic receptors #3 (M3) and autoantibodies against the Muscarinic and Cholinergic receptor #4 M4) are elevated in 20-30% of all patients suffering from ME/CFS. Other studies have shown Beta-1 Adrenergic Receptor Autoantibodies and Beta-2 Adrenergic Receptor Autoantibodies along with Alpha-1 Adrenergic Receptor Autoantibodies, the same autoantibodies which we find in a significant number of patients with Postural Orthostatic Tachycardia Syndrome.
Testing for these autoantibodies is expensive, and it is not proven that immunomodulating therapy or steroids may be effective in these patients although there is some data that low-dose Hydrocortisone does improve patients with ME/CFS. There is also data that B lymphocyte cell depletion with a drug known as Rituximab can result in clinical benefit also. Also, an immunoabsorption technique which removes Beta 2 receptors and depletes them has been shown to be effective. This supports a cause and effect relationship with autoantibodies against receptors and removing them as a clinical response. This improvement in patients has been seen with Chronic Fatigue. In one study, immunoabsorption removed Beta 2 Adrenergic Receptor Antibodies in patients with ME/CFS and showed clinical improvement in memory in symptoms. Some of these patients had long-lasting improvements, while others had short lasting improvements. These are only pilot studies and more research is needed. Other studies have also shown higher autoantibody levels against M1, M3 and M4 Acetylcholine receptors and Beta 2 Adrenergic receptors compared to controls.
Impairments of the Hypothalamic-Pituitary-Adrenal system (considered a portion of the Autonomic Nervous System) have also been reported. There has been noted decrease in Adrenocorticotropic Hormone sensitivity of Adrenal cells expression of negative feedback mechanisms. Some patients with ME/CFS have low Cortisol levels and improvement with low-dose Hydrocortisone has been shown in these patients. In and to hormonal dysregulation, Autonomic dysregulation shows a strong association with ME/CFS. Some studies have shown that more than 90% of patients with ME/CFS have Orthostatic Intolerance. This is strengthened by the fact that many patients with Postural Orthostatic Tachycardia Syndrome (POTS) have similar autoantibodies to patients with ME/CFS. Blood pressure or heart rate regulation abnormalities are seen particularly in adolescents with ME/CFS and many experience symptoms of Orthostatic Intolerance as noted. These patients have worsening symptoms when they get upright posture and improvement when they lie down.
The association of Ehlers-Danlos Syndrome and Autonomic Dysfunction with high frequency of ME/CFS has been intriguing. We believe that there is a genetic predisposition to patients with Ehlers-Danlos Syndrome and Hypermobility spectrum disorders, and they are susceptible to develop Autonomic Dysfunction and Chronic Fatigue after exposure to certain triggers, such as viruses, bacterial infections, emotional stress, trauma, and concussions. Indices of inflammation are also noted to be increased in the populations of patients with ME/CFS. Increased production of various proinflammatory cytokines produce symptoms of fatigue, fevers, adenopathy, myalgias, and arthralgias, sleep disturbances, cognitive impairment and mood disturbances. Infections can trigger or initiate an autoreactive process affecting brain and energy metabolism in people genetically predisposed and patients with abnormal dysbiosis. Patients experience a gradual development of a B cell clone prone to autoregulation, and this may lead to autoimmunity.
Some patients have abnormalities of levels of immunoglobulins. Increased levels of IgA and in some cases, IgM have been noted, and these have been directed against endotoxin components of gram negative bacteria and may be the cause of increased gut permeability noted in many people with ME/CFS.
Exercise is the hallmark treatment for improving patients with ME/CFS. Given that Parasympathetic Excess is a typical Autonomic Dysfunction, usually “low-and-slow” exercise is recommended. Some experts in the field feel patients should exercise no more than two to five minutes at a time followed by five minutes of rest so not to damage skeletal muscle. However, “low-and-slow” exercise, such as walking slowly at no more than 2 mph for 40 minutes, every day for 6 months. No running or jogging or weight lifting or anything else that would raise heart rate too fast. Even if biking or rowing, the motion is still as if walking at no more than 2 mph. This is to re-train the Parasympathetic nervous system to accept small stresses, then larger stresses may be (re-)introduced. For some patients, this is still too stressful. For those days in which a patient simply cannot lift their head off the pillow, supine exercises are recommended, see figure below.
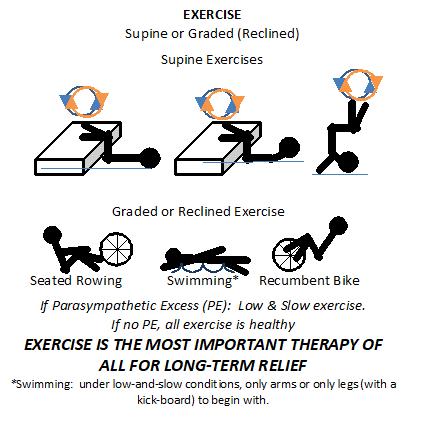
Antimitochondrial cocktails with antioxidants, such as Alpha Lipoic Acid, Coenzyme Q10 and L-carnitine have also been proposed by many experts and some patients are significantly benefited by these cocktails.
In regard to inflammation within the Central Nervous System, there is a glial activation or microglia activation which induces Nitric Oxide and superoxide production of free radicals. These cause neural excitation and neurodegeneration of tissue. Glial activation causes the chronic pain and allodynia in hyperalgesia via the impact a bidirectional signaling mechanism.
In regard to the unrefreshing sleep, we have already discussed the Hypothalamic-Pituitary-Adrenal Axis and the Hypocortisolism. Two meta-analyses have shown an attenuated Cortisol awakening response which may contribute to this morning feeling of non-refreshing sleep.
In addition to exercise and antioxidants, a ketogenic diet, which is high fat and low carbohydrate and limits calorie restriction, or a fasting diet has been recommended. This form of diet has variable results.
Recently from Stanford, a new blood test which produces a stressful environment to white blood cells, in this case mononuclear cells, was developed by Dr. Davis. It appears that patients with ME/CFS have a very high abnormal gradient or electrical charge when exposed to a salt stress environment then cells from normal individuals. Researchers are working arduously to develop these types of test, so we have more objective and easy ways to diagnose ME/CFS. ME/CFS must be differentiated from other entities that have other symptoms which are active participants in causing a malaise, such as collagen vascular disease, cancer, anemia, depression, thyroid disease, drug or pharmacological effects, and other metabolic and infectious diseases.
We believe that mitochondrial mutations or chromosomal mutations in susceptible people may cause ME/CFS. We believe that an autoimmune mechanism may be operative, where in some cases infections induce a normal immune response, but the pathogens may be close enough to our own receptors to cause them to be similarly attacked. After this, additional infections or physical or psychological stress can intensify both the mitochondrial energy deficits and the autoimmunity, and this can create a vicious cycle of fatigue. Patients can present with pain, brain fog, disability and poor exercise tolerance. These are direct or indirect symptoms of Parasympathetic Excess. The association of autoantibodies with similar autoantibodies with POTS and autonomic dysfunction syndrome in ME/CFS patients is not simply coincidence. Note, the Parasympathetic nervous system controls and coordinates the immune system. It may be possible that Parasympathetic Excess causes overactive and persistent immune responses that may lead to autoimmunity. Studies have shown that positive autoimmune tests also show mutations in Mitochondria genes that play an important role in the five mitochondrial respiratory complexes (I, II, III, C & IV; see figure above) in the Electron Transport Chain that produces 90% of the body’s energy with ATP.
The overlap with Hypermobility syndrome, Chronic Fatigue and Autonomic Dysfunction with Orthostatic Intolerance or Parasympathetic Excess states leads us to believe that there is mechanism at the cellular level, which causes an acquired Mitochondria Dysfunction with abnormal energetics producing energy from the body, and that the insulting agents that trigger this are in may cases infectious or inflammatory and can be worsened by emotional stress or trauma stress. They produce a state of inflammation known as oxidative stress which produces energy depleting agents (including oxidants) similar to autoimmunity. Authors have shown that oxidation of critical parts, for example, the Pyruvate Kinase Enzyme System can affectively block the transition of Glycolysis to Aerobic Metabolism, and this demonstrates a biochemical feasibility mechanism. Therefore, the autoimmune model involving the oxidative stress and acquired Mitochondria Dysfunction appear to have significant overlapping features when one looks at all of the studies that have been done on these populations of patients with ME/CFS.
What does this mean in terms of helping the patient? More studies need to be done in terms of using immunomodulating agents in trials, such as IVIG, Corticosteroids and B cell depleting therapies. More work is required assessing the types of exercise programs that are most effective, along with the types of diets that are more effective. The typical American Diet, highly processed foods, full of chemicals, together with the high levels of Psychosocial stress in the American lifestyle may be more of a cause of Chronic Fatigue, than anything else. More is required to study the components and dosages of Mitochondria cocktails that utilize antioxidant agents to see which are most valuable. More work needs to be done to stratify the ME/CFS patients into different phenotypes or categories, as this is a heterogenous group of patients. These patients have different presenting symptoms with different organ systems being more dysfunctional than others.
About the Author
Nicholas L. DePace, MD, FACC is a board-certified cardiologist and Medical Director of Franklin Cardiovascular Associates. A graduate of the Mount Sinai School of Medicine, Dr. DePace has decades of clinical, academic, and research experience and has held faculty appointments as a Clinical Professor of Medicine, becoming one of the youngest full professors in Philadelphia at the time of his appointment.
Dr. DePace specializes in the diagnosis and treatment of autonomic nervous system dysfunction (dysautonomia), including POTS, autonomic dysfunction associated with Ehlers-Danlos syndrome (EDS), chronic fatigue, and anxiety-like conditions that are frequently misdiagnosed. He is nationally recognized for his work on parasympathetic and sympathetic (P&S) nervous system imbalance, a core mechanism underlying many complex chronic disorders.
In addition to treating patients from across the United States, Dr. DePace is a prolific clinical researcher and author of multiple nationally distributed medical textbooks published by Springer and W.W. Norton, focusing on autonomic dysfunction, mitochondrial disorders, cardiovascular disease, and mind–body medicine.


REFERENCES
1 Rasa S, Nora-Krukle Z, Henning N, Eliassen E, Shikova E, Harrer T, Scheibenbogen C, Murovska M, and Prusty BK. Chronic viral infections in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J Translational Med. 2018; 16: 268, doi:10.1186/s12967-018-1644-y.
2 Myhill S, Booth NE, McLaren-Howard J. Chronic fatigue syndrome and mitochondrial dysfunction. Int J Clin Exp Med. 2009; 2(1): 1–16.