Click here to download this post
By Dr. Nicholas L. DePace, M..D., F.A.C.C – Cardiologist specializing in autonomic dysfunction, Ehlers-Danlos syndrome and POTS.
Gastroparesis is a symptomatic disorder of the stomach in which objective evidence of delayed gastric emptying is demonstrated on testing, and symptoms last more than three months and no mechanical obstruction is demonstrated. Oftentimes, an endoscopy is needed to document there is no obstruction or other pathology present which causes slow gastric emptying. Symptoms include nausea and vomiting, early satiety which means that an individual gets full on eating very little, fullness in the abdomen after eating, and abdominal pain and bloating. A Gastroparesis Cardinal Symptom Index scoring system has been developed and may even be used on a daily basis to record symptoms in a scoring system, as gastroparesis symptoms will vary from day to day. A score may be calculated for overall severity of gastroparesis. The daily diary score is known as the GCSI-DD scoring system.
As there is a need for new treatment of gastroparesis, a scoring system such as the GCSI-DD is helpful in following the gastroparesis course and response to treatment. The scoring system involves assessing five symptoms and determining whether there are none, mild, moderate, severe, or very severe in presentation. In terms of vomiting, one generally scores how many episodes of vomiting a person has had in the last 24 hours and gives 0 points for none, 1 point if they vomited once, 2 points if they vomited two times, 3 points for three times or 4 points for four or more episodes of vomiting in a day. Generally, patients with significant gastroparesis will have an average score of at least 3, and we would like to see a reduction of at least 1 point with treatment to be considered effective. A score of 1 is given for mild, 2 for moderate, 3 for severe and 4 points for very severe or intense symptoms for any given item being evaluated. For example, nausea, not able to finish a normal sized meal, feeling excessively full after a meal and upper abdominal pain are four items which are assessed with each item having a score ranging from 0 for none to 4 for very severe. With vomiting, as mentioned, a score of none ranging from none to very severe also goes from 0-4 but is more not involving the severity or intensity of symptoms but rather how many times a patient has vomited in a day. Each of the five items is scored at a maximum of 4 points or 20. If a person has 20 points, an average is taken by dividing by 5 and their score would be 4.0. A normal person who has no symptoms will have a 0 total on all five items.
Once gastroparesis is diagnosed, usually with a delayed gastric emptying with standard gastric emptying studies (although a C-13 isotope breath test may be used, or a wireless capsule motility test may be used) a treatment program is outlined. First, dietary modification is very important. We recommend small meals six times a day, which are low fat and low fiber. We oftentimes try to give fluids which are tolerated better. If an individual cannot tolerate liquids, this is usually a bad prognostic sign. We have patients avoid carbonated beverages, alcohol beverages and tobacco, all of which may worsen gastroparesis. Foods that provoke gastroparesis symptoms are generally fatty, acidic, roughage or spicy. We recommend more bland, sweet, salty and starchy foods. A patient’s diabetic glycemic control is very important although there have not been enough studies demonstrating its effectiveness long-term. At a minimum, we attempt to have a patient consume at least 25 calories times their in weight in kilograms. Carbohydrates are needed. If a patient has difficulty, other measures may have to be employed to get the correct amount of calories, and these include enteral nutrition. Many patients also have concomitant autonomic dysfunction and orthostatic intolerance disorders and require fluids but have difficulty in ingesting the fluid because of nausea, and this is also a problem. Occasionally, a jejunostomy tube needs to placed first, noninvasively through the nose to see if it is effective and subsequently either laparoscopically or surgically in patients as a last resort for those who cannot take ample calories.
Again, oral intake is a preferable route for nutrition and hydration. Restoration of fluids, electrolytes and nutritional support especially in diabetics and optimal glycemic control is extremely important. Pharmacologic agents are a second-line of treatment. There are several categories that are used. These include prokinetic agents, that increase the motility of the stomach and propel food from the stomach to the small intestine, and antiemetic agents, which decrease the nausea that is associated with gastroparesis. Other medications used are antidepressant agents and some investigational therapies. With pharmacologic therapy, usually a combination of a prokinetic or motility medicine is used with an antiemetic drug. Antiemetic drugs are used to control nausea and vomiting in gastroparesis patients.
The first line agent often used is oral Reglan or Metoclopramide. It is a Dopamine-2 receptor agonist. It is the only drug approved by the United States Food and Drug Administration (FDA) for treatment of gastroparesis. However, it is restricted to less than 12 weeks mostly because longer term treatment produces side-effects such as Tardive Dyskinesia in which involuntary movements of the face or body may occur. This is not very common, but when it occurs it may be quite troubling, and the medicine has to be discontinued. Potentially, these may be irreversible, so many patients are afraid to start the medication. However, we see patients start at only 5 mg before meals and generally have not seen any of this develop. Also, acute dystonia which may involve abnormalities of facial muscles and asymmetric distortions of the face, which only occur transiently, may also rarely occur. The incidence of acute dystonia is only 0.2%, which is extremely rare. It is more prevalent in people who receive high doses.
Therefore, we try to keep the dose as low as possible. Metoclopramide may increase an interval on a cardiogram known as the QT interval and, therefore, electrocardiograms are necessary before and during treatment with the medication. Prolongation of the QT interval on an electrocardiogram may predispose to arrhythmias especially if serum electrolytes, such as Potassium or Magnesium become low, and therefore we do monitor electrolytes periodically with these patients also. Metoclopramide is available in different formulations including oral dissolution tablets, oral tablets, and parenteral formulation for people who have flare-ups and need IV medications when hospitalized. With intravenous intake of Metoclopramide we see a higher incidence of dystonic reactions, and this may be reversed with an antihistamine such as Benadryl, which may be given IV or P.O. Also, Benzodiazepines such as Valium may also be given either orally or centrally as may anticholinergic agents such as Benztropine. Four placebo controlled trials have shown good results with Metoclopramide, or Reglan in treating gastroparesis. Gastric emptying was accelerated in all studies in which it was assessed. However, none of the trials were conducted for more than four weeks. It is generally recommended to use the lowest dose of this medication, and liquid forms appear to be more effective because absorption is facilitated. A maximum dose titration is 40 mg a day. Drug holidays are usually helpful or drug reduction at times. One has to be careful with other drugs that interact with the cytochrome P450/2D6 system and someone experienced with prescribing Reglan, usually a motility gastroenterologist, will also be able to look for potential drug interactions or adverse effects when new medicines are added.
Other side-effects of Metoclopramide include fatigue, diarrhea, and feeling restless. Rarely is depression or a condition known as neuroleptic malignant syndrome noted, and this is another reason why longer than 12 weeks of therapy is not required. Data suggested it may not be harmful in pregnancy. We have found this medication useful in patients who have flare-ups and require short courses of intravenous treatment while in the hospital. Also, Metoclopramide is an excellent antiemetic agent to treat nausea and vomiting, even in conditions not associated with gastroparesis, such as radiation sickness, cancer or chemotherapy. It is also effective in migraine syndromes with nausea. It may increase breast milk production in females during lactation.
Patients on antipsychotic medication are recommended to not take Reglan (Metoclopramide). People who have a history of Attention Deficit Disorder, Restless Leg Syndrome, high prolactin levels in the blood, and Parkinson’s disease need to be closely followed on this drug, even for short periods of time. Rarely are blood pressure fluctuations high or low seen with the medication.
The most feared complication is Tardive Dyskinesia which is abnormal involuntary movements of the body, and these are usually seen in people who take the drug for more than three months and at higher dosages. There is a black box warning with chronic or high dose use of the drug.
Metoclopramide has an advantage in that it is a D2 receptor antagonist mechanism, but also has 5-HT3 receptor antagonist and 5-HT4 receptor agonist properties. The 5-HT3 antagonist activity contributes to its antinausea or antiemetic effects. The pro-motility activity of the drug is mediated by a muscarinic activity, D2 receptor antagonist activity and the 5-HT4 receptor agonist activity. Metoclopramide will also increase the tone of the lower esophageal sphincter, and some patients have had beneficial effects with gastroesophageal reflux problems.
Metoclopramide is a drug which passes into the Central Nervous System (CNS). That is, it passes through the Blood-Brain Barrier, and this is one of the reasons there are central side-effects of the drug. Side-effects should be discussed with the patient prior to starting the medicine. As stated, Metoclopramide is approved for diabetic gastroparesis for up 12 weeks durations.
Metoclopramide is a first-line prokinetic therapy and has FDA approval for diabetic gastroparesis. It should be administered at the lowest effective dose and often a liquid formulation is best to facilitate absorption. The major fear is the risk of Tardive Dyskinesia, which may occur but is estimated to be much less than 1%. A patient, if they have any type of involuntary movements should discontinue therapy if they develop side-effects.
Metoclopramide, because of its prokinetic activity, may also enhance gastric antral contractions and decrease postprandial fundus relaxation, which facilitates gastric emptying. Interestingly, it has also been used short term to treat heartburn because it increases the lower esophageal sphincter tone in patients who have used other medications for gastroesophageal reflux and have not had symptom relief. This is also a short-term treatment.
In patients who cannot tolerate, or who do not respond to Metoclopramide, the prokinetic agent next considered by many Gastroenterologists is Domperidone (Motilium). Domperidone’s actions are similar to that of Metoclopramide, which includes stimulation of antral contractions and facilitating the coordination between the antral part of the Stomach and the Duodenum. Although Domperidone does not predominately cross the Blood-Brain Barrier, it does not have the significant central side-effects of Metoclopramide. It may occasionally affect the part of the brain known as the Area Postrema, and it is believed it is here that it exhibits antiemetic properties in addition to its prokinetic or pro-motility properties. Also, since it does not cross the Blood-Brain Barrier, Domperidone is much less likely to cause the involuntary abnormalities of dystonia and Tardive Dyskinesia as seen with Reglan. Breast lactation, headaches and palpitations, however, may occur as side-effects, and Domperidone may also increase the QT interval. Therefore, electrocardiograms have to be done before and after it is instituted. However, review of the literature does not show a significant high incidence of arrhythmias being documented with this agent. Regardless, careful electrocardiogram monitoring is still important.
Domperidone is not FDA approved. It is used in Canada and Europe. It is a better antiemetic than prokinetic medication. It may be obtained through an investigational new drug application with local institutional review boards. Review boards requires patient informed consent to have the medication dispensed from an FDA-authorized pharmacy. Some patients who are extremely sick attempt to obtain it from Canada directly. However, we strongly recommend that they seek the expertise of a Gastrointestinal Motility specialist to prescribe and monitor it.
Domperidone works as a type 2 Dopamine antagonist, therefore, it should not be used at the same time when one is using Reglan. These two drugs should not be used together. It has similar side-effects as Reglan, but as mentioned, less central nervous system side-effects. The starting dose is 10 mg three times a day before meals. It may be increased to 20 mg three times a day before meals and at bedtime. It should not be given if the baseline, QT interval (from the electrocardiograms, or EKG) is greater than 470 ms in males and 450 ms in females. Followup EKGs are needed at least monthly at the start. As with Reglan, drug-on-drug interactions may occur, especially those that affect the CYP2D6 system (part of the cytochrome P450 system). Also, potassium and magnesium levels should be followed in patients who are on this agent. One major study showed that side-effects requiring discontinuation of this drug occurred in only 12% of patients in a large center study of 125 patients. The most common side-effects were headache, tachycardia, palpitations and diarrhea. The majority of patients in this study showed beneficial effects with Domperidone (60%). We have found patients tolerate this medicine well, as long as the QT interval is monitored and does not become unnecessarily prolonged and other medicines which interact with the same enzyme pathways are not being used concomitantly or being used carefully.
Domperidone is excellent for Parkinson’s patients with gastroparesis symptoms. Medicines used for Parkinson disease treatment such as Levodopa may cause nausea side-effects, and drugs such as Metoclopramide, which cross the Blood-Brain Barrier may worsen Parkinson symptoms. Therefore, for associated nausea, Domperidone is an excellent agent in patients with Parkinson’s.
Even though Domperidone may increase motility in Gastroparesis, occasionally symptoms may not necessarily improve, and this demonstrates that increasing the rate of gastric emptying by drugs does not always equate with improving symptoms. Domperidone may also increase lactation and stimulate prolactin production. It is safe for short term use in pregnant women who are lactating and who may need it. It is not approved for this indication in the United States, however. Domperidone has also been shown in studies to be useful and functional for Dyspepsia in both adults and children. Again, this is not approved by the FDA in the US.
Domperidone is contraindicated, as is Metoclopramide, in QT prolongation states in the presence of significant CYP3a4 inhibitors, in the presence of mechanical bowel obstruction, gastrointestinal hemorrhage or bowel perforation with moderate hepatic impairment, or severe renal impairment and in cardiac diseases of significance, especially those with arrhythmias or QT prolongation problems. Erythromycin, often used to increase motility, may increase the drug levels of Domperidone and cause more side-effects since it goes through the CYP3a4 enzyme and is an inhibitor.
Domperidone also increases the lower Esophageal sphincter by blocking Dopamine receptors in the gastric antrum. Domperidone is a selective dopamine D2 and D3 receptor antagonist, but has no clinical intervention with D1 receptors unlike Metoclopramide. It provides relief from nausea by blocking the D2 receptors in the nervous system which medicate nausea, which we have discussed earlier.
Another promotility agent, which was taken off the market, however, is Cisapride (Propulsid). It is available through the investigational limited access program. Five to 10 mg, 15 minutes before meals, and at bedtime is the starting dose, but one may have to go up to 20 mg in some patients. It increases motility in the upper GI and GI tract by directly stimulating 5-HT4 receptor agonists, and is also indirectly a gastroprokinetic drug by acting as a Parasympathetic agonist. That is, the stimulation of Serotonin receptors will increase Acetylcholine release in the Enteric Nervous System which also has a separate effect independent of the autonomic Parasympathetic Nervous System. It was originally used for Gastroesophageal Reflux (GERD). However, it was noted to increase gastric emptying in patients with diabetes. Off-label use for constipation because of its increased motility in the GI tract was also used. It may be through this mechanism that it relieves constipation-like symptoms by indirectly stimulating the release of Acetylcholine in the Muscarinic receptors in the GI tract. In many countries with true Propulsid, because the side-effects included a long QT interval, a warning letter was issued in the past by the US Food and Drug Administration. It was voluntarily withdrawn from the US market in July of 2000 and is not used in many other countries.
Another agent, Tegaserod (Zelnorm) is also a 5-HT4 agonist and has been used for constipation and IBS and has also been shown to be helpful in gastroparesis. The advantage of this agent is it does not have significant QT interval prolongation as Cisapride does. However, enough studies have not been done with this agent. This drug also is not easily obtained. Cisapride is better tolerated than Metoclopramide, but it has important drug interactions with medications and metabolized by the cytochrome P450/3a4 isozyme, and this may result in cardiac arrhythmias. This is why in the United States it may only be obtained through an investigational limited program and if a patient has a QT corrected interval under 450 ms. Tegaserod was temporarily withdrawn from the US market in March 2007. However, it now has restricted use as a treatment investigational new drug protocol. This allows its treatment of IBS and constipation or chronic idiopathic constipation. It is restricted to use in patients who have no known preexisting heart disease.
Recently, a medication, Prucalopride (Prudac), a selective, high affinity 5-HT4 receptor agonist has been shown to improve motility in chronic constipation. It was approved by Europe in 2009, Canada in 2011 and in Israel in 2014. It was recently approved by the FDA in this county. Physicians have used it off-label in gastroparesis because of its favorable pharmacokinetics and its low interaction potential with other medications. It is also known as Motegrity. It is the only Serotonin-4 receptor agonist for adults with chronic idiopathic constipation available now in the United States. A study in the American Journal of Gastroenterology, published in August 2019, involved Prucalopride in gastroparesis and was a randomized controlled study. Thirty-four patients with gastroparesis, 28 of whom are idiopathic, were enrolled in this study. A double-blind crossover trial of four weeks was undertaken. Symptom severity scoring was used in this study. A Carbon-13 breath test was used to assess gastric emptying studies. Compared with placebo, Prucalopride significantly improved the total Gastroparesis Cardinal Symptom Index with improved nausea and abdominal symptom scoring. The gastric half-emptying time was significantly enhanced by this agent compared to placebo. Quality of life improved. More studies are needed to see if longer than four weeks treatment will still continue these beneficial effects, as this may become a valuable agent in the treatment of gastroparesis in the future.
Another motility drug that is probably the most potent motility drug is Erythromycin. However, it is poorly tolerated and oftentimes causes nausea, which is one of the things we are attempting to treat with gastroparesis. It is, however, effective in the hospital when people have acute flare-ups and given intravenously. One of the problems with Erythromycin, or any of the Macrolide antibiotics that are given, is that it may increase the QT interval, and there has been a question of people having increased mortality on these types of medications in various analyses of data. Given orally, Erythromycin improves gastric emptying and symptoms for several weeks, but eventually the body does get used to it and it is not as effective; a term we call tachyphylaxis, due to downregulation of the Motilium receptors for which this drug acts on. Therefore, its clinical responsiveness dropout rate occurs at about four weeks of oral treatment people. It also has significant drug interactions with the CYP3a4 system. It may increase the QT corrected interval also and should not be given to patients with prolonged QT values on their baseline EKG. We have found it useful given short term in patients with flare-ups of gastroparesis. There have not been a significant amount of studies done with either Reglan or Domperidone in combination with Erythromycin possibly because of the QTc interval prolongation issue. Interestingly, as mentioned, it causes nausea but also because of abdominal pain it may mimic the symptoms we are trying to treat with gastroparesis which makes its effectiveness difficult to evaluate at times.
Erythromycin has been used in patients with diabetic gastroparesis, idiopathic gastroparesis and postvagotomy surgical gastroparesis. As mentioned, it is probably the most potent gastroparesis motility agent when used intravenously and sometimes is used to clear the stomach from food contents prior to an endoscopy. One study found it equivalent to Metoclopramide for short term improvement in gastroparesis symptoms. One should be careful giving Calcium Blockers concomitantly with Erythromycin since the both interfere with the P-450 system.
While it may appear to be an advantage to give dual prokinetic therapy, these have not been studied, and again the possible enhancing of QT prolongation makes it more of an arrhythmia risk and the risk/benefit ratio has to be weighed.
Antiemetic agents are frequently helpful in gastroparesis because of the nausea and vomiting. Agents used have been phenothiazines, antihistamines, anticholinergics, and Dopamine receptor antagonist, and more recently Serotonin receptor antagonists. Phenothiazines appear to be work through a central anti-Dopamine mechanism. Commonly used agents are Compazine, Tigan and Phenergan.
Antiemetic agents are usually used as second-line agents behind prokinetic drugs such as Metoclopramide. These medicines all carry side-effects such as QT prolongation. One commonly used is Ondansetron (Zofran) which is a 5-HT3 receptor antagonist. However, studies do not show it is superior to Metoclopramide when given in the emergency department. Anticholinergic drugs such as tricyclics (Amitriptyline and Nortriptyline) are also used in low dose as antiemetic agents. At higher dose, however, then may have significant anticholinergic effects and may worse gastroparesis motility symptoms and should be only used at low doses. Generally, we do not use antiemetic drugs unless patients fail motility drugs first. It is often best to give medicines such as Zofran on a p.r.n. or as needed basis. Side-effects with Zofran may occur, such as constipation, skin rashes and headaches.
Other antiemetic drugs that are being evaluated in patients with gastroparesis include Neurokinin receptor antagonists (i.e., Emend). These medicines have been effective in chemotherapy-induced nausea and vomiting. A four-week short-term study has shown an improvement in the GCSI scores for gastroparesis. Of note, Promethazine is an antihistamine, which may also increase the QTc interval on EKG and is also restricted in use for nausea.
Off-label cannabis is used oftentimes for nausea in a gastroparesis patient. It is commonly known as Marijuana. There is also a synthetic cannabinoid known as Dronabinol (Marinol). However, there is a risk of Hyperemesis or increased vomiting with withdraw of these medicines, and also with daily use of Marijuana one may also get Hyperemesis syndrome if they are even on the medicine every day, which may mimic gastroparesis. These symptoms include nausea, vomiting and abdominal pain. If these occur, one should quit Cannabis immediately.
Transdermal Scopolamine has been used empirically off-label for nausea and has been effective in some patients. This patch is often used for prophylaxis of sea sickness.
Tricyclics, as mentioned, may sometimes be helpful for nausea and definitely helpful for abdominal pain in low dose. Nortriptyline is less anticholinergic than Amitriptyline and may be somewhat safer in gastroparesis especially at low doses. We have found it specifically helpful in nausea with pain. The 5-HT2 receptor antagonist, Mirtazapine (Remeron) may also be effective in nausea and gastroparesis patients.
Because of abdominal pain, oftentimes people will seek narcotics. These actually may worsen gastroparesis by impairing GI motility and are not recommended. Tramadol may be used in low doses and is a narcotic antagonist, which may be effective. If possible, however, try to use Gabapentin, Pregablin or tricyclics such as Nortriptyline for the abdominal pain in gastroparesis.
There are patients who are refractory to all types of treatment and cannot even take in sufficient calories and fluids. There are attempts at using Endoscopic Botulism to improve Pyloric obstruction that have been somewhat disappointing. There have been studies in these types of patients with this technique. Surgical endoscopy approaches may be considered for people who are refractory to drug treatments as may gastroelectric stimulation, a sort of pacemaker which is placed in the stomach. All of these options are usually for people with severe refractory gastroparesis. Gastric electrical stimulation (GES) improves symptoms mostly in diabetic patients and is listed as a “compassionate” treatment. Oftentimes a gastric stimulating tube has been placed via a nasogastric route. If the patient responds, then a more definitive procedure may be done. Of course, infection is always a complication.
Gastroelectric stimulation involves the implantation of a neurostimulator. This delivers a high frequency low energy signal into the GI tract. Usually two electrode leads are placed. They are placed within the gastric muscle. Usually there is a generator and in a subcutaneous abdominal pouch. Besides infection, occasionally patients may feel a shocking sensation. Usually symptoms of nausea and vomiting improve with these implanted devices. However, abdominal pain does not improve. Diabetic patients appear to improve more than idiopathic gastroparesis subsets although both types of patients may improve.
A prospective analysis of 151 patients at a single center showed that gastroelectrical stimulation improved symptoms in 75% of patients and 43% were at least moderately improved [[i]]. Diabetic patients were more improved than nondiabetic patients. The NICE guidelines on gastric electrical stimulators (GES) for gastroparesis were upgraded in 2015 from 2004. In 2004, the guidelines stated that the current safety and efficacy of this procedure did not appear to be supported. However, they went on to say in 2014 “since there was a considerable amount of new evidence available and NICE has updated the guidance in May 2014 and now states that current evidence on the efficacy and safety of gastric electrical stimulation for gastroparesis is adequate to support the use of the procedure with normal arrangements for clinical governance-consent and audit. During the consent process, clinicians should inform patients considering gastroelectric stimulation for gastroparesis that some patients do not get any benefit from it. They should also give patients detailed written information about the risk of complications, which may be serious including the need to remove the device.” They go into detail about how GES have been reported to enhance nutritional status and reduce requirements for supplemental feeds and improve glycemic control in diabetics. Patient selection is important. Expertise in gastrointestinal motility disorders should be sought out by specialized centers and surgeons who work in these units.
The NICE guidelines recommend that patients that have severe nausea and vomiting occurring at an average of at least once daily and who are proven refractory to aggressive antiemetic and prokinetic drug therapy for at least one year in duration should be candidates for GES. Patients who have nausea and vomiting and who are not on narcotics appear to respond better. A recent study in 2008 of 119 patients receiving GES, (64 diabetics and 55 idiopathic) had the devices placed laparoscopically. After a followup of 34, plus or minus 27 months in diabetics and 44 plus or minus 26 months in idiopathic patients, improvement was seen. This is the largest long-term followup of up to over three years. No mortalities were device related. A total of 18 patients died during the study group, and they were mostly diabetic. Diabetes had the greatest mortality rate (25%).
The GCSI scores improved and use of prokinetic and narcotic medicines decreased significantly at greater than one year with high satisfaction rates. Patients who were able to decrease the use of prokinetic and narcotic medications achieved long-term satisfaction. However, we need to emphasize the diabetic patients who developed severe gastroparesis had a high mortality rate over time. These patients should be followed very closely perhaps for cardiac or vascular causes of death during that time period, and gastroparesis may be a marker of a more generalized state where a patient is a high risk of death from cardiac or vascular complications. General autonomic testing to assess for Cardiac Autonomic Neuropathy may be done in the office as may noninvasive tests, such as stress test and echocardiography for risk stratification. Risk factor reduction should be undertaken more aggressively in these patients, and they should be followed more closely. The diabetic population of gastroparesis is a high-risk patient population for mortality based on these study results. The battery life is approximately 4-5 years with GES devices. They may be removed at any time.
Some studies show up to 20% of patients may experience complications from gastric pacer implants, or GES implants. These include infection, migration, erosion of the stimulation device, stomach wall perforation, pain due to adherence bands from pacing wires connected to the abdominal wall, dislodgement, breakage and erosion leads in the small bowel. Laparoscopic means of putting it in is a more noninvasive approach.
Temporary endoscopic stimulation in a gastroparesis-like syndrome has also shown beneficial results. Gastroparesis-like syndrome patients have symptoms similar to gastroparesis but do not have delayed gastric emptying. One study utilized 551 patients who were suffering from symptoms of gastroparesis. This study showed that at 2 hours gastric retention decreased for the delayed patients, which is a beneficial effect and it actually increased in patients with normal or rapid gastric emptying, which was also a beneficial effect. These changes were accompanied by improvement of vomiting, nausea and total symptom score in all three subgroups. The authors concluded that gastroelectrical stimulation may be an effective therapy treatment in symptoms of gastroparesis with normal gastric emptying or rapid gastric emptying. Further studies on treating gastroparesis-like symptoms with non-delayed gastric emptying are needed.
As patients become more refractory to treatment of gastroparesis, surgical techniques may also be considered. As they need treatment, feeding with Jejunostomy for nutritional support with a Jejunostomy tube that bypasses the stomach is effective. Oftentimes, a venting gastrostomy tube is required, at the same time, to relieve distention from the stomach. These may be performed surgically or endoscopically. Some patients have been treated aggressively with gastric bypass with gastrojejunostomy, and this has been used in some specialized centers. Rarely is partial gastrectomy done, but if a person has a postsurgical gastroparesis, occasional complete gastrectomy is performed, and this requires a significant amount of expertise at a major center.
Pyloromyotomy surgically or laparoscopically has also been used. Studies have shown improvement with Pyloromyotomy in patients in terms of gastric emptying and reduction and need for prokinetic therapy three months post surgery. Of course, surgery is a more invasive route, and some expert centers prefer to use GES, which is reversible, and may be a less invasive option compared to gastric surgery for treatment of patients with chronic drug-refractory nausea and vomiting secondary to gastroparesis. GES may actually represent an intermediated step between the more or less invasive treatments. Interestingly, GES may override gastric-type arrhythmias in the stomach and stimulate gastric emptying and eliminate symptoms.
In the case of surgical procedures, the mechanism of how Pyloroplasty is effective is not known. It may depend on the residual antral motor function in the stomach. Recently, the safety and feasibility of oral Pyloromyotomy performed non-surgically through the mouth after prior GES for gastroparesis was published [[ii]]. In this study, 38% of the patients were diabetic and 62% were idiopathic. The average length of GES insertion was 3.45 years. There was significant GCSI improvement with a reduction of 1.63 points, as the patients had symptomatic improvement. Both symptoms and motility significantly improved in the short-term. Therefore, if a patient fails both conventional and pharmacologic therapy and eventually GES therapy, Peroral Pyloromyotomy may be quite effective. It also has a low complication rate and is fairly noninvasive.
One study reviewed patients undergoing GES, pyloric surgery, and combined GES and pyloric surgery. The combination of GES and combined pyloric surgery appeared to have significantly improved nausea and vomiting. The patients who benefited the most were those with more than nausea and vomiting than abdominal pain. A meta-analysis looking at Peroral Endoscopic Pyloromyotomy demonstrated clinical success in treating refractory gastroparesis. In this study, interestingly, idiopathic gastroparesis prior treatment with Botulinum injection and a GES appeared to be a positive predictive effect on the four hour gastric emptying study results after a Gastric Peroral Endoscopy.
In summary, Endoscopic Botulism may be attempted but results have not been predictable. Some patients may respond while many will not. The GES, or gastric pacemaker may be placed and may initially work, and then eventually a patient may become refractory to it after several years and still benefit from a surgical Pyloromyotomy, many of which may be done noninvasively orally. Combinations of GES and Pyloromyotomy are options in patients who are severely symptomatic.
As further research is ongoing, treatment appears to be encouraging in that there appear to be more and more options from lifestyle changes to pharmacology, to gastric pacemakers and surgical procedures that may be done and many of them in combination to relieve patients of symptoms.
Some more investigational treatments are undergoing active research. One is a new drug called Relamorelin. Another is TZP-102, a novel second generation Ghrelin receptor agonist, which is believed to enhance the motility of the GI tract. Octreotide, is a long-acting synthetic analogue of endogenous somatostatin that inhibits growth hormones. Glucagon and insulin have been shown to decrease pooling of blood in the enteric circulation and enhance gastric emptying and reduces early satiety. As mentioned, prior, Botulism injection into the Pylorus is also undergoing further research.
Lastly, we would like to comment overall on the autonomic dysfunction and its association with gastroparesis. Autoimmune disorders, which produce an Enteric Ganglionitis, may occasionally occur and destroy the enteric or intestinal neurons. They are associated with anti-Hu antibodies directed against nuclear structures of neuronal cells. There are other antibodies directed against calcium and potassium channels, antibodies against acetylcholine receptors, and antibodies against parietal cells to name a few. Diabetic gastroparesis could be due to autonomic neuropathy. Heart rate variability measurements have been done successfully for diagnosis of autonomic neuropathy, and this is noninvasive test that may be done. Immune mechanisms may be operative. Autoimmune autonomic ganglionopathy is a disorder of isolated autonomic failure associated with antibodies to nicotinic acetylcholine receptors of autonomic ganglia which results in severe orthostatic intolerance, syncope, constipation, gastroparesis, urinary retention, dry mouth, dry eyes, blurred vision, and anhidrosis. The higher antibody titers appear to correlate with widespread dysautonomia whereas lower antibody levels may be seen in people with just one organ system representative.
Also, patients with autoimmune dysautonomia and gastroparesis may have antibodies to glutamic acid decarboxylase. In these patients, immunomodulatory therapy improved symptoms in small numbers of patients positive for antibodies against Glutamic Acid Decarboxylase who were refractory to drug and device therapies. Therefore, the possibility of some of the gastroparesis disorders, whether they are diabetic or idiopathic, may have accompanying autonomic dysfunction and autoimmune components. Furthermore, immunomodulating therapy, such as IVIG, prednisone, and other agents may occasionally be helpful. Significant research needs to be done on this, and certainly many gastroparesis patients have undergone autoimmune testing.
The most conventional algorithm at the present time for patients with gastroparesis symptoms is to identify if they have delayed gastric emptying. There are accepted protocols for several of the tests we have described, but a gastric emptying study using a standard meal and nuclear scintography appears to be the most widespread in use. First-line therapy should be diet. Prokinetic therapy would be second-line therapy. Oftentimes, third-line therapy would be antiemetic therapy, which oftentimes may be combined with prokinetic therapy, but QT prolongation has to be carefully followed and sought for in the electrocardiogram. Patients who are refractory may need a feeding Jejunostomy and a Decompressive Gastrostomy to get enteral feeding nutrition, fluid and hydration. If the patients may eat sufficiently, consideration for patients who have failed aggressive pharmacological therapy should include discussion for Gastroelectrical Stimulators (GES) and perhaps surgical procedures including Endoscopic Pyloromyotomy. Again, we cannot emphasize enough the need to go to a specialized Gastric Motility Center, which has experience with all of these modalities.
About the Author
Nicholas L. DePace, MD, FACC is a board-certified cardiologist and Medical Director of Franklin Cardiovascular Associates. A graduate of the Mount Sinai School of Medicine, Dr. DePace has decades of clinical, academic, and research experience and has held faculty appointments as a Clinical Professor of Medicine, becoming one of the youngest full professors in Philadelphia at the time of his appointment.
Dr. DePace specializes in the diagnosis and treatment of autonomic nervous system dysfunction (dysautonomia), including POTS, autonomic dysfunction associated with Ehlers-Danlos syndrome (EDS), chronic fatigue, and anxiety-like conditions that are frequently misdiagnosed. He is nationally recognized for his work on parasympathetic and sympathetic (P&S) nervous system imbalance, a core mechanism underlying many complex chronic disorders.
In addition to treating patients from across the United States, Dr. DePace is a prolific clinical researcher and author of multiple nationally distributed medical textbooks published by Springer and W.W. Norton, focusing on autonomic dysfunction, mitochondrial disorders, cardiovascular disease, and mind–body medicine.


REFERENCES
________________________
[1] Heckert J, Sankineni A, Hughes WB, Harbison S, Parkman H. Gastric Electric Stimulation for Refractory Gastroparesis: A Prospective Analysis of 151 Patients at a Single Center. Dig Dis Sci. 2016 Jan;61(1):168-75. doi: 10.1007/s10620-015-3837-z. Epub 2015 Aug 18.
[2] Strong AT, Rodriguez J, Kroh M, Ponsky J, Cline M, El-Hayek K. Safety and Feasibility of Per-Oral Pyloromyotomy as Augmentative Therapy after Prior Gastric Electrical Stimulation for Gastroparesis. J Am Coll Surg. 2019 Dec;229(6):589-595. doi: 10.1016/j.jamcollsurg.2019.09.014. Epub 2019 Oct 11.





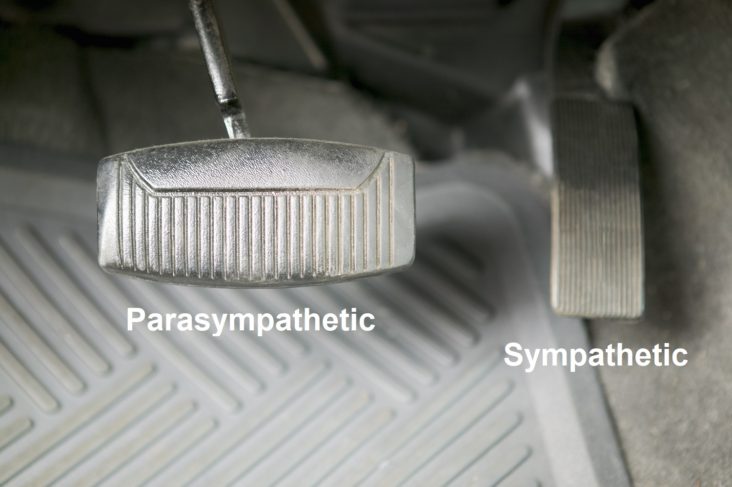

 between the two (SB = S/P, known as Sympathovagal Balance) that is the key. Again with the car analogy, even if you have no brakes and no accelerator (you are very old or very sick) you may still roll down hill; even then if you cannot stop you crash. A normal ratio of Sympathetic to Parasympathetic is approximately 1.0 (SB = 1.0 is perfect balance). If SB is high, indicating that the Sympathetics are much more reactive than the Parasympathetics, this may exaggerate or amplify all Sympathetic responses. For example, little stimuli may become painful, little stresses may cause anxiety, little allergic reactions may become rashes or hives (significant histamine reactions). Insufficient Parasympathetic activity with excessive Sympathetic activity (a typical result of persistent stress, including psychosocial stress) may suppress the immune system, over stimulate the production of oxidants leading to excessive oxidative stress, raise blood pressure, promote atherosclerosis, cause persistent inflammation, accelerate diabetes, promote atherosclerosis, and accelerate the onset of heart disease, kidney disease, or dementia.
between the two (SB = S/P, known as Sympathovagal Balance) that is the key. Again with the car analogy, even if you have no brakes and no accelerator (you are very old or very sick) you may still roll down hill; even then if you cannot stop you crash. A normal ratio of Sympathetic to Parasympathetic is approximately 1.0 (SB = 1.0 is perfect balance). If SB is high, indicating that the Sympathetics are much more reactive than the Parasympathetics, this may exaggerate or amplify all Sympathetic responses. For example, little stimuli may become painful, little stresses may cause anxiety, little allergic reactions may become rashes or hives (significant histamine reactions). Insufficient Parasympathetic activity with excessive Sympathetic activity (a typical result of persistent stress, including psychosocial stress) may suppress the immune system, over stimulate the production of oxidants leading to excessive oxidative stress, raise blood pressure, promote atherosclerosis, cause persistent inflammation, accelerate diabetes, promote atherosclerosis, and accelerate the onset of heart disease, kidney disease, or dementia. normal function of aging, it is a risk indicator and the risk is significantly higher if the SB is abnormal, especially if SB is high indicating Sympathetic Excess. Ways to keep the sympathetic nervous system from becoming overactive or excessive include lifestyle changes, such as meditation, yoga, Tai Chi, or other forms of mild to moderate exercise. Various exercises can train the sympathetic nervous system not to become overactive and may also be good stress reducers.
normal function of aging, it is a risk indicator and the risk is significantly higher if the SB is abnormal, especially if SB is high indicating Sympathetic Excess. Ways to keep the sympathetic nervous system from becoming overactive or excessive include lifestyle changes, such as meditation, yoga, Tai Chi, or other forms of mild to moderate exercise. Various exercises can train the sympathetic nervous system not to become overactive and may also be good stress reducers. with POTS or orthostatic hypotension disorders) we generally begin with recumbent exercises, such as a recumbent bicycle, a rowing machine, or swimming (see insert, left). In the worst cases we recommend stating with exercises that including lying on the floor with your feet up on the bed or couch or the like and moving your lower legs like you are walking (see insert, left). In fact, a rowing machine is probably the best exercise initially for patients with
with POTS or orthostatic hypotension disorders) we generally begin with recumbent exercises, such as a recumbent bicycle, a rowing machine, or swimming (see insert, left). In the worst cases we recommend stating with exercises that including lying on the floor with your feet up on the bed or couch or the like and moving your lower legs like you are walking (see insert, left). In fact, a rowing machine is probably the best exercise initially for patients with  volume. Stroke volume is the amount of blood your heart pumps with each beat. Stroke volume is very important, since patients with these disorders often have low stroke volumes which means their hearts are not pumping enough blood to the brain while you are upright (sitting or standing).
volume. Stroke volume is the amount of blood your heart pumps with each beat. Stroke volume is very important, since patients with these disorders often have low stroke volumes which means their hearts are not pumping enough blood to the brain while you are upright (sitting or standing).