Click here to download this post
By Dr. Nicholas DePace and Dr. Joseph Columbo
Long COVID – Cardiac Involvement
Common cardiac problems may occur with labile heart rate and blood pressure responses to activity.
Myocarditis and pericarditis may occur chronically. In the acute stages, myocardial infarction, cardiac failure, life-threatening arrhythmias, and sudden cardiac death have been described.
The incidents of arrhythmias in Long COVID syndrome are unknown, but many individuals have palpitations, and studies using ambulatory monitoring need to be further conducted.
Sequelae from acute COVID may occur, such as coronary artery aneurysm, aortic aneurysm, atherosclerosis, and venous and arterial thrombotic disease including life-threatening pulmonary embolism.
These structural abnormalities may manifest itself in Long-COVID syndrome long after recovery of acute illness and predispose to arrhythmias, breathlessness, and acute coronary events, such as heart attacks and chest pain syndromes.
Myocardial injury is the most common abnormality detected with acute COVID infection. It is usually detected even when patients with no cardiac symptoms demonstrate elevated cardiac troponin levels, which may be evident in a high percentage of patients with COVID-19 [69].
Further research is ongoing as to whether this myocardial injury pattern, even when subclinical, may lead to increased arrhythmias and heart failure in the long-term.
Echocardiographic studies have shown abnormalities with COVID-19, including right ventricular dysfunction 26.3%, left ventricular dysfunction 18.4%, diastolic dysfunction 13.2%, and pericardial perfusion 7.2%.
To what extent this is reversible in patients who go on to Long-COVID syn drome is not known. In addition, sleep abnormalities and difficulties that reduce quality of life have been noted in Long-COVID syndrome patients.
These may also adversely affect cardiac function, provoke arrhythmias, elevate blood pressure, and exacerbate or cause hypertensive states. Chest pain and palpitations are status post-acute phase of COVID-19.
Palpitations were reported in 9% and chest pain in 5% of patients 6 months after follow-up. To track heart inflammation, one of the most effective and sensitive tests is cardiac magnetic resonance imaging (MRI).
Inflammation rates may be as high as 60% more than 2 months after a diagnosis of COVID, although this is a very difficult test to obtain in many centers that do not have it readily available.
Long-COVID syndrome patients may present with chest pain in 17% of patients, palpitations in 20% of patients, and dyspnea on exertion 30% of patients.
The question of myocarditis is always raised especially in children, but adults are also known to have myocarditis.
One small study showed that in healthy college athletes with mild COVID-19 symptoms, 15% had evidence of MRI findings consistent with myocarditis on a screening study.
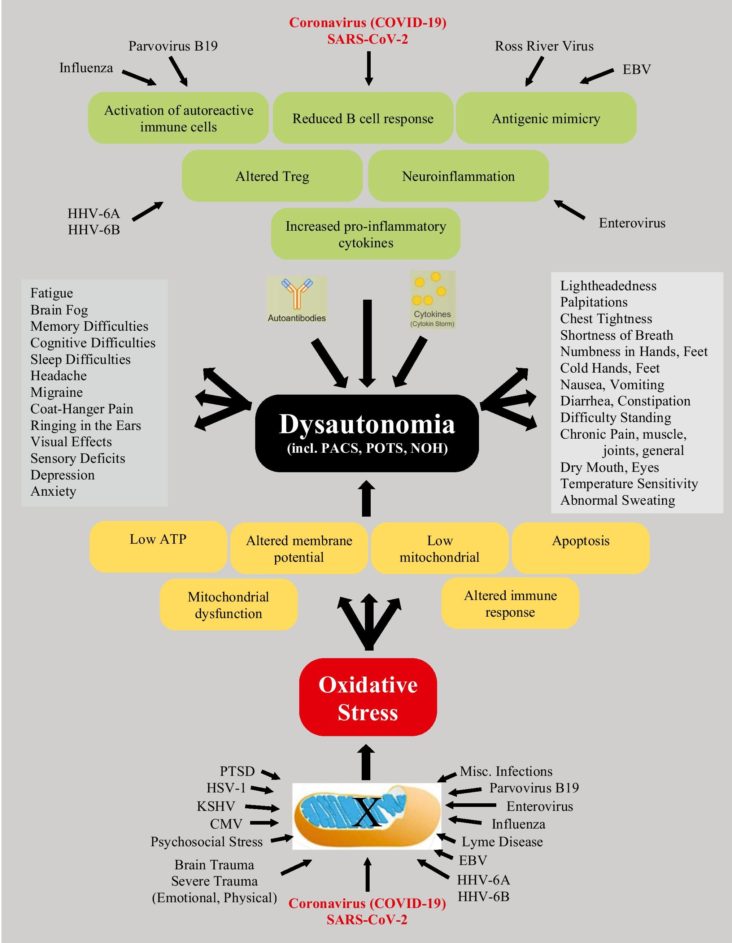
More importantly, many of the chest pains and palpitations, which appear to be cardiology in etiology, are actually due to autonomic dysfunction, including the postural orthostatic tachycardia syndrome (POTS).
Therefore, the importance of not only doing cardiac imaging, ambulatory monitoring, stress testing, 6-min walk test, echocardiography, and other noninvasive cardiac workup, but also autonomic Current Cardiology Reports 1 3 testing, such as cardiorespiratory monitoring, HRV interval testing, beat-to-beat blood pressure with tilt testing, and sudomotor testing may be useful in diagnosing autonomic nervous dysfunction. Arrhythmias are noted with Long COVID, but attention to the use of anti-arrhythmic drugs, amiodarone, for example, must be used carefully in patients who have fibrotic pulmonary changes after COVID-19.
This Post is an excerpt from Current Cardiology Reports: https://link.springer.com/article/10.1007/s11886-022-01786-2