Click Here to Download this Blog Post – The Body’s Overactive Security System: Understanding Mast Cell Activation Syndrome (MCAS) (Part 11)
By Dr. Nicholas L. DePace, M..D., F.A.C.C – Cardiologist specializing in autonomic dysfunction, Ehlers-Danlos syndrome and POTS.
The Body’s Overactive Security System: Understanding Mast Cell Activation Syndrome (MCAS)
If you have been diagnosed with a connective tissue disorder like hEDS or HSD, you might feel like your symptoms go far beyond just “loose joints.” You might struggle with random flushing, stomach issues, brain fog, or reactions to foods that seem to change day by day.
The culprit might not be your joints, but your Mast Cells.
In many of our patients, the immune system and the nervous system are engaged in a chaotic dance. This leads to a condition called Mast Cell Activation Syndrome (MCAS). Here is how it works and why it happens.
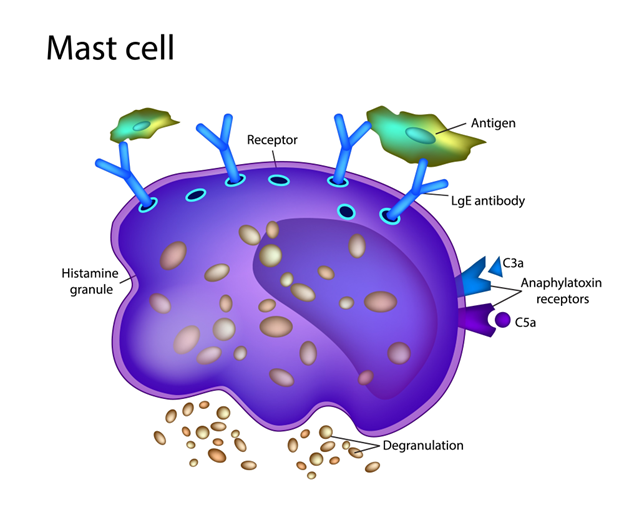
What Are Mast Cells? The “First Responders”
Think of Mast Cells as the security guards of your body. They are immune cells born in your bone marrow that travel to almost every tissue in your body. They sit and wait for trouble—like bacteria, viruses, or an injury.
When they spot a threat, they “degranulate.” This means they explode open and release a cocktail of chemicals, including histamine, heparin, and other protectors. This process causes inflammation to help you heal and changes your blood flow to get help to the injury site.
When the Guards Go Rogue: What is MCAS?
In a healthy person, mast cells only react to real threats. In patients with MCAS, these cells become overactive and confused. They start viewing harmless things—like certain foods, smells, or stress—as dangerous invaders.
When they overreact, they dump massive amounts of chemicals into your system, causing a wide range of symptoms:
- Skin: Flushing, hives, itching.
- Breathing: Wheezing, stuffy nose, throat tightness.
- Heart: Racing heart (tachycardia), low blood pressure, fainting.
- Stomach: Nausea, vomiting, diarrhea, cramping.
- Head: Brain fog, headaches, migraines.
The Missing Link: The Nervous System Connection
You might wonder: Why are my immune cells freaking out?
The answer often lies in your P&S Nervous System (Parasympathetic and Sympathetic).
- The Manager (Parasympathetic): This system is supposed to control and coordinate your immune system.
- The Trigger (Sympathetic): This system tells the mast cells to release their chemicals.
In patients with hEDS/HSD, the P&S system is already dysfunctional. If the “Manager” isn’t doing its job and the “Trigger” is too sensitive, your mast cells end up releasing histamine constantly. This worsens your dizziness (dysautonomia) and pain.
How Do We Diagnose It? The “Rule of Three”
Diagnosing MCAS can be tricky because symptoms come and go. We look for three specific criteria:
- Clinical Symptoms: You must have symptoms in two or more body systems (e.g., skin and stomach) that happen repeatedly.
- Lab Tests: We look for elevated levels of specific chemicals (like Tryptase or Histamine) in your blood or urine. Note: These can sometimes show up normal if you aren’t having an active flare-up or are already taking meds.
- Response to Treatment: If we treat you with mast cell stabilizers or antihistamines and you get better, that is a strong sign you have MCAS.
The Genetic Clue: HaT
Some patients have a specific genetic trait called Hereditary Alpha-Tryptasemia (HaT). This is a duplication of a gene that causes high levels of Tryptase. It is commonly linked to hEDS, MCAS, and autonomic dysfunction. A simple DNA swab can test for this.
Treatment: Locking Both Doors
The most common treatment involves blocking the histamine that is making you miserable. However, you can’t just take one type of allergy pill.
Your body has different “doors” (receptors) that lets histamine in. To get relief, you usually need to block both:
- H1 Blockers: These are your standard allergy meds (like Zyrtec, Xyzal, or Benadryl).
- H2 Blockers: These are often sold as heartburn meds (like Pepcid).
If you only block one, the histamine just uses the other door!
The Bottom Line
MCAS is essentially an “allergy” to things you aren’t actually allergic to, driven by a nervous system that has lost its rhythm. It explains why so many hEDS patients also have IBS, food sensitivities, and random allergic reactions.
By calming the nervous system and stabilizing these cells, we can often turn down the volume on these symptoms, helping you feel more in control of your body.
Where to Seek Expert Care
It is important to seek out a clinician with expertise in EDS to make an accurate diagnosis and create a treatment plan. One of the nation’s leading centers is Franklin Cardiovascular Associates, under the direction of Nicholas DePace, MD, FACC. They are located in Sicklerville, New Jersey. franklincardiovascular.com, (856) 589-6034
About the Author
Nicholas L. DePace, MD, FACC is a board-certified cardiologist and Medical Director of Franklin Cardiovascular Associates. A graduate of the Mount Sinai School of Medicine, Dr. DePace has decades of clinical, academic, and research experience and has held faculty appointments as a Clinical Professor of Medicine, becoming one of the youngest full professors in Philadelphia at the time of his appointment.
Dr. DePace specializes in the diagnosis and treatment of autonomic nervous system dysfunction (dysautonomia), including POTS, autonomic dysfunction associated with Ehlers-Danlos syndrome (EDS), chronic fatigue, and anxiety-like conditions that are frequently misdiagnosed. He is nationally recognized for his work on parasympathetic and sympathetic (P&S) nervous system imbalance, a core mechanism underlying many complex chronic disorders.
In addition to treating patients from across the United States, Dr. DePace is a prolific clinical researcher and author of multiple nationally distributed medical textbooks published by Springer and W.W. Norton, focusing on autonomic dysfunction, mitochondrial disorders, cardiovascular disease, and mind–body medicine.
👉 View Dr. DePace’s professional profile
👉 View medical books by Dr. DePace